
The authors have designated this AOP as all rights reserved. Re-use in any form requires advanced permission from the authors.
AOP: 584
Title
SQLE accumulation leads to hepatotoxicity via cholesterol/sphingolipid metabolic disturbance and lysosomal damage
Short name
Graphical Representation
Point of Contact
Contributors
- Peihua Luo
Coaches
OECD Information Table
| OECD Project # | OECD Status | Reviewer's Reports | Journal-format Article | OECD iLibrary Published Version |
|---|---|---|---|---|
This AOP was last modified on July 10, 2025 01:52
Revision dates for related pages
| Page | Revision Date/Time |
|---|
Abstract
Drug-induced liver injury (DILI) describes unintended hepatic damage caused by pharmacological agents. As the primary metabolic organ, the liver exhibits heightened susceptibility to drug toxicity. Numerous clinical agents induce hepatotoxicity by disrupting metabolic homeostasis, particularly lipid, bile acid, and nucleoside metabolism. Nevertheless, upstream molecular mechanisms linking metabolic dysregulation to hepatotoxicity are incompletely defined.
Hepatotoxic agents include small molecule kinase inhibitors (SMKIs), a distinct pharmacological class. Certain compounds reportedly induce hepatotoxicity via hepatic cholesterol biosynthesis disruption. Crizotinib, an ALK tyrosine kinase inhibitor and first-line therapy for ALK-positive NSCLC, is associated with clinical reports of severe hepatic complications and steatosis.
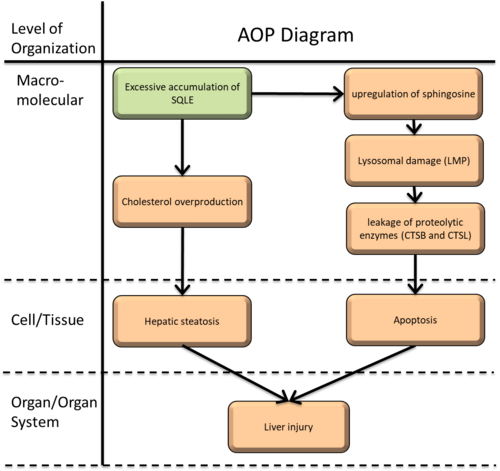
Drawing from previous research, a specific adverse outcome pathway (AOP) has been identified, potentially mediated by excessive accumulation of SQLE, likely offering insights into specific molecular mechanisms contributing to hepatotoxicity and drug-induced abnormal lipid metabolism. In this AOP, the Molecular Initiating Event (MIE) is " Excessive accumulation of SQLE," triggering six Key Events (KEs): " Upregulation of sphingosine," " Lysosomal damage (LMP)," " leakage of proteolytic enzymes (CTSB and CTSL)" " Cholesterol overproduction," "Hepatic steatosis, " and "Hepatocyte apoptosis," Ultimately, this pathway culminates in the Adverse Outcome (AO) of Liver injury.
AOP Development Strategy
Context
Strategy
Summary of the AOP
Events:
Molecular Initiating Events (MIE)
Key Events (KE)
Adverse Outcomes (AO)
Relationships Between Two Key Events (Including MIEs and AOs)
Network View
Prototypical Stressors
Life Stage Applicability
Taxonomic Applicability
Sex Applicability
Overall Assessment of the AOP
Domain of Applicability
Essentiality of the Key Events
Evidence Assessment
Known Modulating Factors
| Modulating Factor (MF) | Influence or Outcome | KER(s) involved |
|---|---|---|
Quantitative Understanding
Considerations for Potential Applications of the AOP (optional)
References
- Yan H, Huang X, Zhou Y, Mu Y, Zhang S, Cao Y, Wu W, Xu Z, Chen X, Zhang X, Wang X, Yang X, Yang B, He Q, Luo P. Disturbing Cholesterol/Sphingolipid Metabolism by Squalene Epoxidase Arises Crizotinib Hepatotoxicity. Adv Sci (Weinh). 2025 Apr;12(14): e2414923.
- Andrade, R.J., Chalasani, N., Björnsson, E.S. et al. Drug-induced liver injury. Nat Rev Dis Primers 5, 58 (2019).
- Li K, Deng Y, Deng G, Chen P, Wang Y, Wu H, Ji Z, Yao Z, Zhang X, Yu B, Zhang K. High cholesterol induces apoptosis and autophagy through the ROS-activated AKT/FOXO1 pathway in tendon-derived stem cells. Stem Cell Res Ther. 2020 Mar 20;11(1):131.
- Sozen E, Demirel-Yalciner T, Koroglu MK, Elmas MA, Ercan F, Ozer NK. High cholesterol diet activates ER stress mediated apoptosis in testes tissue: Role of α-tocopherol. IUBMB Life. 2022 Jan;74(1):85-92.
- Ogretmen B. Sphingolipid metabolism in cancer signalling and therapy. Nat Rev Cancer. 2018 Jan;18(1):33-50.
- Kuo A, Hla T. Regulation of cellular and systemic sphingolipid homeostasis. Nat Rev Mol Cell Biol. 2024 Oct;25(10):802-821. doi: 10.1038/s41580-024-00742-y. Epub 2024 Jun 18.