
This Event is licensed under the Creative Commons BY-SA license. This license allows reusers to distribute, remix, adapt, and build upon the material in any medium or format, so long as attribution is given to the creator. The license allows for commercial use. If you remix, adapt, or build upon the material, you must license the modified material under identical terms.
Event: 2293
Key Event Title
Aromatase, induction
Short name
Biological Context
| Level of Biological Organization |
|---|
| Molecular |
Cell term
| Cell term |
|---|
| eukaryotic cell |
Organ term
| Organ term |
|---|
| gonad |
Key Event Components
| Process | Object | Action |
|---|---|---|
| aromatase activity | aromatase | increased |
Key Event Overview
AOPs Including This Key Event
| AOP Name | Role of event in AOP | Point of Contact | Author Status | OECD Status |
|---|---|---|---|---|
| Aromatase induction leading to estrogen receptor alpha activation via increased estradiol | MolecularInitiatingEvent | Martina Panzarea (send email) | Under development: Not open for comment. Do not cite |
Taxonomic Applicability
Life Stages
| Life stage | Evidence |
|---|---|
| All life stages |
Sex Applicability
| Term | Evidence |
|---|---|
| Unspecific |
Key Event Description
Biological state
Aromatase (synonyms: Cytochrome P450 aromatase, CYP19) plays a central role in steroidogenesis by converting androgens to estrogens through a three-step reaction that allows the aromatization of the A-ring of the steroid molecule (Mendelson et al., 1985; Thompson and Siiteri, 1974; Simpson and Santen, 2015) (see Fig. 1, Caciolla et al.2020). It is considered the rate-limiting enzyme in estrogen biosynthesis (Simpson and Santen, 2015; Zhao et al., 2016) since it catalyzes the final and key step, i.e., the conversion of C19 steroids to estrogen (Bulun et al., 2005). More specifically, aromatase converts through aromatization androstenedione and testosterone to estrone (E1) and estradiol (E2), respectively. Aromatase has been proposed as an important molecular target for many environmental endocrine disruptors chemicals (Laville et al., 2006).
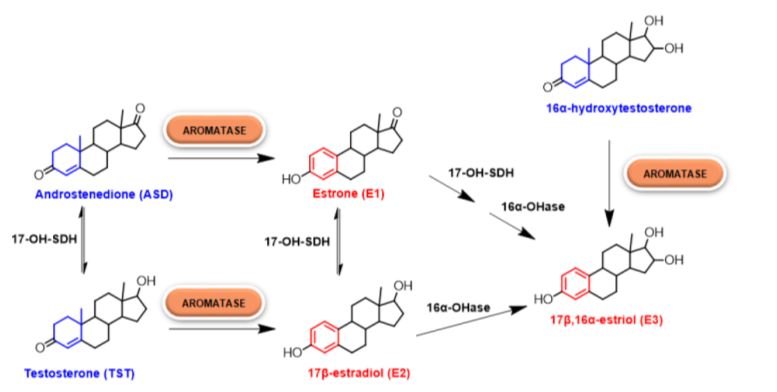
Figure 1. Estrogen biosynthesis. In particular, the role of human aromatase is highlighted (Caciolla et al., 2020)
Biological compartment
The enzyme is present in all vertebrates as the product of expression of a single gene, with some exceptions represented by pigs and teleosts, where duplication events have produced three and two isoforms, respectively. The protein is expressed in different tissues in vertebrates, where it plays an essential role in reproductive biology as estrogens are responsible for ovarian differentiation, development of the reproductive system, sex differentiation, and reproduction. Moreover, a critical role of estrogens has also been demonstrated in brain, bone, skin, fat, and cardiovascular tissues (Di Nardo et al., 2021). In the normal endometrium aromatase is not expressed (Bulun 2009; Zhao et al., 2016).
General role in biology
Estrogen is synthesized by aromatase in the gonads and in several extragonadal organs, such as skin, adipose tissues, liver, heart and brain (Bulun et al., 2005; Simpson 2003, Bulun et al., 2009). Extragonadal estrogen is biologically active in a paracrine or intracrine fashion, although it may escape the local metabolism and enter the circulation (Simpson and Davis, 2001; Simpson, 2003).
In premenopausal women, ovary is the primary source of estrogens (primarily in the granulosa cells and corpus luteum), and the cyclic expression of estrogen by the ovaries drives endometrial proliferation (Mihm et al., 2011). Disease-free endometrium lacks aromatase and thus does not produce estrogen locally (Bulun 2009; Zhao et al., 2016).
In postmenopausal women, peripheral tissues, especially adipose tissue, become the main site of estrogen synthesis (Davis et al., 2015). The estrogen precursor androstenedione is primarily secreted by the adrenal glands (Zhao et al., 2016), and estrogen (E1, E2) is produced in many extragonadal organs (skin, adipose tissues, liver, heart and brain) (Bulun et al., 2005). After menopause, adipocytes, preadipocytes, and mesenchymal stem cells within fat tissue are the predominant source of aromatase, the enzyme responsible for the conversion of androgens to estrogen E1 and, to a lesser extent E2 (Zhao et al., 2016).
How It Is Measured or Detected
Expression:
Aromatase mRNA expression can be measured by RT-PCR [in JEG-3 choriocarcinoma cells cultures (Laville et al., 2006); in H295R Human Adrenocortical Carcinoma Cells (Sanderson et al., 2002); in KGN Human Ovarian Granulose-Like Tumor Cell Line (Morinaga et al., 2004)] and in situ hybridization [endometrial carcinoma tissue samples (Segawa et al., 2005)]
Aromatase protein levels can be measured by Western blot from cell cultures and liver microsomal samples (You et al., 2001) and by immunohistochemistry on tissue sections (You et al., 2001, Segawa et al., 2005)
Activity:
The catalytic aromatase activity was determined by using the tritium release assay (measurement of tritium released after the conversion of tritium-labelled androstenedione into estrone by cells or hepatic microsomes as described previously (Drenth et al., 1998; Lephart and Simpson, 1991) with minor modifications [in JEG-3 choriocarcinoma cells cultures (Laville, 2006); in H295R Human Adrenocortical Carcinoma Cells (Sanderson et al., 2002); in hepatic microsomal samples (You et al., 2001); in KGN Human Ovarian Granulose-Like Tumor Cell Line (Morinaga et al., 2004)].
Indirect methods:
Levels of estrogens in serum can offer an indirect measurement of aromatase induction.
Domain of Applicability
Aromatase levels and activity increase as a function of age and adiposity (Simpson et al., 1987; Bulun and Simpson, 1994) and, therefore, contribute to estrogen-induced endometrial proliferation in the postmenopausal woman (Blakemore and Naftolin, 2016; Zhao et al., 2016). Consistent with the role of adipose tissue in estrogen synthesis, obesity is more strongly associated with the development of endometrial cancer than any other cancer type in women (Reeves et al., 2007).
In the mouse aromatase is expressed in fewer tissues (gonads, brain) than human aromatase; thus, mouse models do not mirror estrogen production in humans. Aromatase is only present in gonads, brain and male gonadal fat in mice (Zhao et al., 2016). Generation of humanized aromatase (Aromhum) mouse model that contains the full human aromatase gene, allowed to mimic human aromatase expression pattern in the mouse model (Zhao et al., 2012). In the rat, in addition to expression in gonads and brain, aromatase was induced after ovariectomy in liver and subcutaneous adipose tissue (Zhao et al., 2005)
Biological domains of applicability
- Taxonomic applicability: Vertebrates. Invertebrates of the genus Branchiostoma (Di Nardo et al., 2021).
- Life stage applicability: All life stages, mainly adulthood (UniProt)
- Sex applicability: Males, females.
Evidence for the biological domain of applicability
Aromatase converts, through aromatization, androstenedione and testosterone to estrone (E1) and estradiol (E2), respectively. Aromatase induction could increase circulating estrogens (E1, E2) available to estrogenic activation pathways in estrogenic sensitive tissues (uterus).
References
Blakemore J and Naftolin F, 2016. Aromatase: Contributions to Physiology and Disease in Women and Men. Physiology (Bethesda), 31:258-269. doi: 10.1152/physiol.00054.2015
Bulun SE, Lin Z, Zhao H, Lu M, Amin S, Reierstad S and Chen D, 2009. Regulation of aromatase expression in breast cancer tissue. Ann N Y Acad Sci, 1155:121-131. doi: 10.1111/j.1749-6632.2009.03705.x
Bulun SE and Simpson ER, 1994. Regulation of aromatase expression in human tissues. Breast Cancer Research and Treatment, 30:19-29. doi: 10.1007/BF00682738
Bulun SE, Lin Z, Imir G, Amin S, Demura M, Yilmaz B, Martin R, Utsunomiya H, Thung S, Gurates B, Tamura M, Langoi D and Deb S, 2005. Regulation of aromatase expression in estrogen-responsive breast and uterine disease: from bench to treatment. Pharmacol Rev, 57:359-383. doi: 10.1124/pr.57.3.6
Caciolla J, Bisi A, Belluti F, Rampa A and Gobbi S, 2020. Reconsidering Aromatase for Breast Cancer Treatment: New Roles for an Old Target. Molecules, 25. doi: 10.3390/molecules25225351
Davis SR, Lambrinoudaki I, Lumsden M, Mishra GD, Pal L, Rees M, Santoro N and Simoncini T, 2015. Menopause. Nat Rev Dis Primers, 1:15004. doi: 10.1038/nrdp.2015.4
Di Nardo G, Zhang C, Marcelli AG and Gilardi G, 2021. Molecular and Structural Evolution of Cytochrome P450 Aromatase. Int J Mol Sci, 22. doi: 10.3390/ijms22020631
Drenth H-J, Bouwman CA, Seinen W and Van den Berg M, 1998. Effects of Some Persistent Halogenated Environmental Contaminants on Aromatase (CYP19) Activity in the Human Choriocarcinoma Cell Line JEG-3. Toxicology and Applied Pharmacology, 148:50-55. doi: https://doi.org/10.1006/taap.1997.8307
Laville N, Balaguer P, Brion F, Hinfray N, Casellas C, Porcher J-M and Aït-Aïssa S, 2006. Modulation of aromatase activity and mRNA by various selected pesticides in the human choriocarcinoma JEG-3 cell line. Toxicology, 228:98-108. doi: https://doi.org/10.1016/j.tox.2006.08.021
Lephart ED and Simpson ER, 1991. Assay of aromatase activity. Methods Enzymol, 206:477-483. doi: 10.1016/0076-6879(91)06116-k
Mendelson CR, Wright EE, Evans CT, Porter JC and Simpson ER, 1985. Preparation and characterization of polyclonal and monoclonal antibodies against human aromatase cytochrome P-450 (P-450AROM), and their use in its purification. Arch Biochem Biophys, 243:480-491. doi: 10.1016/0003-9861(85)90525-9
Mihm M, Gangooly S and Muttukrishna S, 2011. The normal menstrual cycle in women. Anim Reprod Sci, 124:229-236. doi: 10.1016/j.anireprosci.2010.08.030
Morinaga H, Yanase T, Nomura M, Okabe T, Goto K, Harada N and Nawata H, 2004. A benzimidazole fungicide, benomyl, and its metabolite, carbendazim, induce aromatase activity in a human ovarian granulose-like tumor cell line (KGN). Endocrinology, 145:1860-1869. doi: 10.1210/en.2003-1182
Reeves GK, Pirie K, Beral V, Green J, Spencer E and Bull D, 2007. Cancer incidence and mortality in relation to body mass index in the Million Women Study: cohort study. BMJ, 335:1134. doi: 10.1136/bmj.39367.495995.AE
Sanderson JT, Boerma J, Lansbergen GW and van den Berg M, 2002. Induction and inhibition of aromatase (CYP19) activity by various classes of pesticides in H295R human adrenocortical carcinoma cells. Toxicol Appl Pharmacol, 182:44-54. doi: 10.1006/taap.2002.9420
Segawa T, Shozu M, Murakami K, Kasai T, Shinohara K, Nomura K, Ohno S and Inoue M, 2005. Aromatase Expression in Stromal Cells of Endometrioid Endometrial Cancer Correlates with Poor Survival. Clinical Cancer Research, 11:2188-2194. doi: 10.1158/1078-0432.Ccr-04-1859
Simpson, E., & Santen, R. J. (2015). Celebrating 75 years of oestradiol. Journal of Molecular Endocrinology, 55(3), T1-T20. Retrieved Dec 19, 2024, from https://doi.org/10.1530/JME-15-0128
Simpson ER, 2003. Sources of estrogen and their importance. The Journal of Steroid Biochemistry and Molecular Biology, 86:225-230. doi: https://doi.org/10.1016/S0960-0760(03)00360-1
Simpson ER and Davis SR, 2001. Minireview: Aromatase and the Regulation of Estrogen Biosynthesis—Some New Perspectives. Endocrinology, 142:4589-4594. doi: 10.1210/endo.142.11.8547
Simpson ER and Mendelson CR, 1987. Effect of aging and obesity on aromatase activity of human adipose cells. Am J Clin Nutr, 45:290-295. doi: 10.1093/ajcn/45.1.290
Thompson E.A., Siiteri P.K. Utilization of oxygen and reduced nicotinamide adenine dinucleotide phosphate by human placental microsomes during aromatization of androstenedione. J. Biol. Chem. 1974;249:5364–5372. doi: 10.1016/S0021-9258(20)79735-8
You L, Sar M, Bartolucci E, Ploch S and Whitt M, 2001. Induction of hepatic aromatase by p,p'-DDE in adult male rats. Mol Cell Endocrinol, 178:207-214. doi: 10.1016/s0303-7207(01)00445-2
Zhao H, Tian Z, Hao J and Chen B, 2005. Extragonadal aromatization increases with time after ovariectomy in rats. Reprod Biol Endocrinol, 3:6. doi: 10.1186/1477-7827-3-6
Zhao H, Pearson EK, Brooks DC, Coon JSt, Chen D, Demura M, Zhang M, Clevenger CV, Xu X, Veenstra TD, Chatterton RT, DeMayo FJ and Bulun SE, 2012. A humanized pattern of aromatase expression is associated with mammary hyperplasia in mice. Endocrinology, 153:2701-2713. doi: 10.1210/en.2011-1761
Zhao H, Zhou L, Shangguan AJ and Bulun SE, 2016. Aromatase expression and regulation in breast and endometrial cancer. J Mol Endocrinol, 57:R19-33. doi: 10.1530/jme-15-0310