This Key Event Relationship is licensed under the Creative Commons BY-SA license. This license allows reusers to distribute, remix, adapt, and build upon the material in any medium or format, so long as attribution is given to the creator. The license allows for commercial use. If you remix, adapt, or build upon the material, you must license the modified material under identical terms.
Relationship: 302
Title
Reduction, Testosterone synthesis by ovarian theca cells leads to Reduction, 17beta-estradiol synthesis by ovarian granulosa cells
Upstream event
Downstream event
Key Event Relationship Overview
AOPs Referencing Relationship
| AOP Name | Adjacency | Weight of Evidence | Quantitative Understanding | Point of Contact | Author Status | OECD Status |
|---|---|---|---|---|---|---|
| Androgen receptor agonism leading to reproductive dysfunction (in repeat-spawning fish) | adjacent | High | Low | Dan Villeneuve (send email) | Open for citation & comment | WPHA/WNT Endorsed |
Taxonomic Applicability
Sex Applicability
| Sex | Evidence |
|---|---|
| Unspecific | Not Specified |
Life Stage Applicability
| Term | Evidence |
|---|---|
| Adult, reproductively mature | Moderate |
Key Event Relationship Description
Evidence Collection Strategy
Evidence Supporting this KER
Biological Plausibility
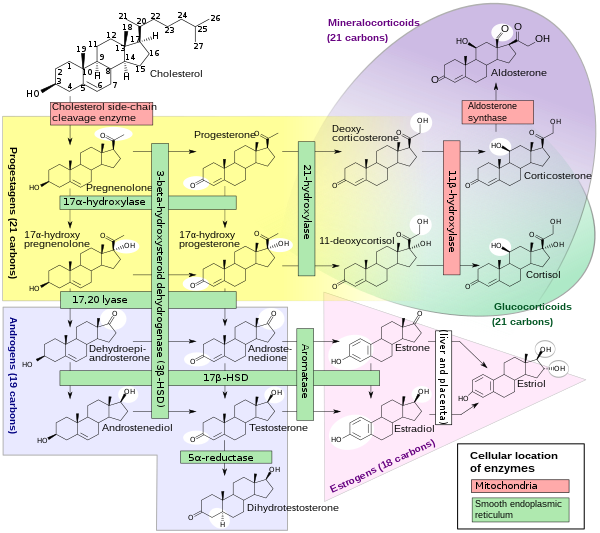
Theca cell-derived androgens (e.g., testosterone, androstenedione) are precursors for estrogen (e.g., 17β-estradiol, estrone) synthesis. Androgens secreted from the theca cells are aromatized to estrogens in the ovarian granulosa cells (Norris 2007; Senthilkumaran et al. 2004). Consequently, reductions in theca cell testosterone synthesis can be expected to reduce the rate of estradiol synthesis by the ovarian granulosa cells (Payne and Hales 2004; Miller 1988; Nagahama et al. 1993).
Empirical Evidence
- Ex vivo T production by ovary tissue collected from female fathead minnows exposed in vivo to 33 or 472 ng 17β-trenbolone/L was significantly reduced after 24 or 48 h of exposure (Ekman et al. 2011). Reductions in ex vivo T production preceded significant reductions in ex vivo E2 production.
- Ketoconazole is a fungicide thought to inhibit CYP11A and CYP17 (both involved in theca cell androgen production) with greater potency than it inhibits CYP19 (aromatase) (Villeneuve et al. 2007). Ex vivo E2 and T production were significantly reduced following exposure to 30 or 300 μg ketoconazole/L (Ankley et al. 2012).
Uncertainties and Inconsistencies
No significant inconsistencies identified to date. However, the literature review on this topic has not been comprehensive.
Known modulating factors
Quantitative Understanding of the Linkage
At present we are unaware of any well established quantitative relationships between ex vivo T production (as an indirect measure of theca cell T synthesis) and ex vivo E2 production (as an indirect measure of granulosa cell E2 synthesis). There are considerable data available which might support the development of such a relationship. Additionally, there are a number of existing mathematical/computational models of ovarian steroidogenesis that may be adaptable to support a quantitative understanding of this linkage (Breen et al. 2007; Shoemaker et al. 2010; Quignot and Bois 2013).
Response-response Relationship
Time-scale
Known Feedforward/Feedback loops influencing this KER
Domain of Applicability
Enzymes required for testosterone and 17ß-estradiol synthesis are only found in vertebrates and amphioxus (Markov et al. 2009; Baker 2011). They are not present in invertebrates. Consequently, this KER is not applicable to invertebrates.
References
-
Ankley GT, Cavallin JE, Durhan EJ, Jensen KM, Kahl MD, Makynen EA, et al. 2012. A time-course analysis of effects of the steroidogenesis inhibitor ketoconazole on components of the hypothalamic-pituitary-gonadal axis of fathead minnows. Aquatic toxicology 114-115: 88-95.
- Baker ME. 2011. Origin and diversification of steroids: Co-evolution of enzymes and nuclear receptors. Mol Cell Endocrinol 334: 14-20.
- Breen MS, Villeneuve DL, Breen M, Ankley GT, Conolly RB. 2007. Mechanistic computational model of ovarian steroidogenesis to predict biochemical responses to endocrine active compounds. Annals of biomedical engineering 35(6): 970-981.
- Ekman DR, Villeneuve DL, Teng Q, Ralston-Hooper KJ, Martinovic-Weigelt D, Kahl MD, et al. 2011. Use of gene expression, biochemical and metabolite profiles to enhance exposure and effects assessment of the model androgen 17beta-trenbolone in fish. Environmental toxicology and chemistry / SETAC 30(2): 319-329.
- Markov GV, Tavares R, Dauphin-Villemant C, Demeneix BA, Baker ME, Laudet V. Independent elaboration of steroid hormone signaling pathways in metazoans. Proc Natl Acad Sci U S A. 2009 Jul 21;106(29):11913-8. doi: 10.1073/pnas.0812138106.
- Miller WL. 1988. Molecular biology of steroid hormone synthesis. Endocrine reviews 9(3): 295-318.
- Nagahama Y, Yoshikumi M, Yamashita M, Sakai N, Tanaka M. 1993. Molecular endocrinology of oocyte growth and maturation in fish. Fish Physiology and Biochemistry 11: 3-14.
- Norris DO. 2007. Vertebrate Endocrinology. Fourth ed. New York: Academic Press.
- Payne AH, Hales DB. 2004. Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocrine reviews 25(6): 947-970.
- Quignot N, Bois FY. 2013. A computational model to predict rat ovarian steroid secretion from in vitro experiments with endocrine disruptors. PloS one 8(1): e53891.
- Senthilkumaran B, Yoshikuni M, Nagahama Y. A shift in steroidogenesis occurring in ovarian follicles prior to oocyte maturation. Mol Cell Endocrinol. 2004 Feb 27;215(1-2):11-8.
- Shoemaker JE, Gayen K, Garcia-Reyero N, Perkins EJ, Villeneuve DL, Liu L, et al. 2010. Fathead minnow steroidogenesis: in silico analyses reveals tradeoffs between nominal target efficacy and robustness to cross-talk. BMC systems biology 4: 89.
- Villeneuve DL, Ankley GT, Makynen EA, Blake LS, Greene KJ, Higley EB, et al. 2007. Comparison of fathead minnow ovary explant and H295R cell-based steroidogenesis assays for identifying endocrine-active chemicals. Ecotoxicol Environ Saf 68(1): 20-32.