
The authors have designated this AOP as all rights reserved. Re-use in any form requires advanced permission from the authors.
AOP: 261
Title
L-type calcium channel blockade leading to heart failure via decrease in cardiac contractility
Short name
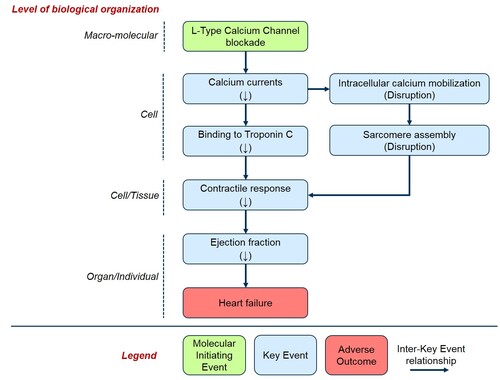
Graphical Representation
Point of Contact
Contributors
- Luigi Margiotta-Casaluci
Coaches
OECD Information Table
| OECD Project # | OECD Status | Reviewer's Reports | Journal-format Article | OECD iLibrary Published Version |
|---|---|---|---|---|
| 1.45 | Under Development |
This AOP was last modified on April 29, 2023 16:03
Revision dates for related pages
| Page | Revision Date/Time |
|---|---|
| Blockade, L-Type Calcium Channels | November 01, 2018 12:25 |
| Decrease, Calcium currents | June 19, 2018 14:19 |
| Decrease, Calcium binding to Troponin C | June 19, 2018 13:58 |
| Decrease, Cardiac contractility | June 19, 2018 14:02 |
| Decrease, Cardiac ejection fraction | June 19, 2018 14:03 |
| Heart failure | December 03, 2024 10:15 |
| Disruption, Intracellular calcium mobilization | June 21, 2018 04:46 |
| Disruption, Sarcomere assembly | June 21, 2018 04:46 |
| Blockade, L-Type Calcium Channels leads to Decrease, Calcium currents | June 19, 2018 13:54 |
| Decrease, Calcium currents leads to Decrease, Calcium binding to Troponin C | June 19, 2018 14:04 |
| Decrease, Calcium binding to Troponin C leads to Decrease, Cardiac contractility | June 19, 2018 14:04 |
| Decrease, Cardiac contractility leads to Decrease, Cardiac ejection fraction | June 19, 2018 14:05 |
| Decrease, Cardiac ejection fraction leads to Heart failure | June 19, 2018 14:08 |
| Decrease, Calcium currents leads to Disruption, Intracellular calcium mobilization | June 21, 2018 04:48 |
| Disruption, Intracellular calcium mobilization leads to Disruption, Sarcomere assembly | June 21, 2018 04:48 |
| Disruption, Sarcomere assembly leads to Decrease, Cardiac contractility | June 21, 2018 04:48 |
| Calcium channel blockers | June 21, 2018 04:57 |
| Nifedipine | November 02, 2018 07:40 |
| Amlodipine | November 02, 2018 07:41 |
| Felodipine | November 02, 2018 07:41 |
| Nisoldipine | November 02, 2018 07:42 |
| Nimodipine | November 02, 2018 07:42 |
| Nitrendipine | November 02, 2018 07:43 |
| Diltiazem | November 02, 2018 07:43 |
| Verapamil | November 29, 2016 18:42 |
Abstract
Calcium ions play a vital role in cellular and organism physiology. These ions are a central component of a complex system of intracellular messenger that mediates a wide range of biological processes. In the heart, calcium dynamics and signaling are essential for cardiac muscle contraction, thus the perturbation of these processes may impair organ function and health. Different types of calcium channels contribute to the timely regulation of calcium currents at the cellular level. Among them, the L-type calcium channels (LTCCs) are responsible for the excitation-contraction coupling of skeletal, smooth, and cardiac muscle. The pharmacological modulation of this target is an important tool for the treatment of cardiac pathologies, and several drugs that block LTCCs have been developed in the last few decades. However, pharmaceuticals (or any other chemical) that unintentionally block this channel in cardiac cells may lead to adverse cardiovascular effects.
This AOP describes the mechanistic linkage between LTCCs blockade and heart failure mediated by the decrease in cardiac contractility and reduction of ejection fraction. This AOP should be considered part of a network, together with AOP 262. The AOP development was based on over 1,100 in vitro, ex vivo and in vivo data points extracted from approximately 150 publications that investigated a) the effects of calcium channel blockers on different components of the cardiovascular system, and b) the effects of genetic manipulations of the drug target. For each Key Event (KE), a quantitative analysis was performed to determine effect direction and degree of responsiveness. A database-specific confidence index was assigned to each KE according to the degree of reproducibility of the effect. Many KEs of this AOP can be experimentally quantified using different specific endpoints. Therefore, the responsiveness analysis of each endpoint was performed to quantify the sensitivity of each measurement to the blockade of LTCCs. This knowledge has both biological and methodological significance. In the latter case, it can effectively inform the development of suitable testing strategies aimed at maximizing the probability to detect changes in a given KE. Several in silico methods are currently available to determine the quantitative relationship between various KEs in the two proposed AOPs in humans. Future development efforts will be aimed at combining those different methods to develop a fully quantitative AOP network. This AOP is intended to support the interpretation of the cardiovascular risk associated with drug-induced blockade of LTCCs.
AOP Development Strategy
Context
Cardiovascular safety liabilities remain a major cause of drug attrition during preclinical and clinical development. Drug-induced cardiovascular toxicity was identified as an area of potential interest for AOP development by a network of experts convened by the UK National Centre for the Replacement, Refinement, and Reduction of Animals in Research (NC3Rs) and the European Union Reference Laboratory for alternatives to animal testing (EURL-ECVAM) in 2015. The blockade of L-type calcium channels (LTCCs) was proposed as one of the priority molecular initiating events (MIE) that may benefit from the AOP vision.
From a toxicological perspective, the key importance of ion currents for drug safety came to light with the discovery that hERG potassium channel inhibition is the most common mechanism of drug-induced long QT syndrome and torsades de pointes arrhythmia [PMID:16322774]. However, a growing body of research has demonstrated that drugs can affect more cardiac currents than previously expected (e.g. calcium current). Thus, the integrated assessment of drug inhibitory activity for multiple ion currents may provide a more accurate prediction of the toxicological risk [PMID:16322774]. Considering the newly recognized complexity of the phenomenon, several authors called for dedicated inter-disciplinary efforts aimed at improving our understanding of the mechanistic basis of cardiovascular liabilities, beyond hERG current blockade [PMID: 21306581]. This AOP is intended to support those efforts by providing a detailed map of the multi-scale effects mediated by LTCCs-blockade.
Strategy
Summary of the AOP
Events:
Molecular Initiating Events (MIE)
Key Events (KE)
Adverse Outcomes (AO)
| Type | Event ID | Title | Short name |
|---|
| MIE | 1529 | Blockade, L-Type Calcium Channels | Blockade, L-Type Calcium Channels |
| KE | 1530 | Decrease, Calcium currents | Decrease, Calcium currents |
| KE | 1531 | Decrease, Calcium binding to Troponin C | Decrease, Calcium binding to Troponin C |
| KE | 1532 | Decrease, Cardiac contractility | Decrease, Cardiac contractility |
| KE | 1533 | Decrease, Cardiac ejection fraction | Decrease, Cardiac ejection fraction |
| KE | 1536 | Disruption, Intracellular calcium mobilization | Disruption, Intracellular calcium mobilization |
| KE | 1537 | Disruption, Sarcomere assembly | Disruption, Sarcomere assembly |
| AO | 1535 | Heart failure | Heart failure |
Relationships Between Two Key Events (Including MIEs and AOs)
| Title | Adjacency | Evidence | Quantitative Understanding |
|---|
| Blockade, L-Type Calcium Channels leads to Decrease, Calcium currents | adjacent | High | High |
| Decrease, Calcium currents leads to Decrease, Calcium binding to Troponin C | adjacent | High | High |
| Decrease, Calcium binding to Troponin C leads to Decrease, Cardiac contractility | adjacent | High | High |
| Decrease, Cardiac contractility leads to Decrease, Cardiac ejection fraction | adjacent | High | High |
| Decrease, Cardiac ejection fraction leads to Heart failure | adjacent | High | High |
| Decrease, Calcium currents leads to Disruption, Intracellular calcium mobilization | adjacent | High | Moderate |
| Disruption, Intracellular calcium mobilization leads to Disruption, Sarcomere assembly | adjacent | Moderate | Low |
| Disruption, Sarcomere assembly leads to Decrease, Cardiac contractility | adjacent | High | Low |
Network View
Prototypical Stressors
Life Stage Applicability
| Life stage | Evidence |
|---|---|
| All life stages | High |
Taxonomic Applicability
| Term | Scientific Term | Evidence | Link |
|---|---|---|---|
| Vertebrates | Vertebrates | High | NCBI |
Sex Applicability
| Sex | Evidence |
|---|---|
| Male | High |
| Female | High |