
This AOP is licensed under the BY-SA license. This license allows reusers to distribute, remix, adapt, and build upon the material in any medium or format, so long as attribution is given to the creator. The license allows for commercial use. If you remix, adapt, or build upon the material, you must license the modified material under identical terms.
AOP: 292
Title
Inhibition of tyrosinase leads to decreased population in fish
Short name
Graphical Representation
Point of Contact
Contributors
- Young Jun Kim
Coaches
- Dan Villeneuve
OECD Information Table
| OECD Project # | OECD Status | Reviewer's Reports | Journal-format Article | OECD iLibrary Published Version |
|---|---|---|---|---|
| 1.78 | Under Development |
This AOP was last modified on April 29, 2023 16:03
Revision dates for related pages
| Page | Revision Date/Time |
|---|---|
| Inhibition of tyrosinase | May 03, 2019 08:28 |
| Reduction of L-Dopaquinone | May 03, 2019 08:29 |
| Reduction in melanin level | May 03, 2019 08:30 |
| Reduction of melanosome level | May 03, 2019 08:31 |
| Reduction fo Pigmentation pattern | May 03, 2019 08:31 |
| Decrease, Population growth rate | January 03, 2023 09:09 |
| tyrosinase leads to Reduction of L-Dopaquinone | May 03, 2019 08:33 |
| Reduction of L-Dopaquinone leads to Reduction in melanin level | May 03, 2019 08:33 |
| Reduction in melanin level leads to Reduction of melanosome level | May 03, 2019 08:35 |
| Reduction of melanosome level leads to Reduction fo Pigmentation pattern | May 03, 2019 08:35 |
| Reduction fo Pigmentation pattern leads to Decrease, Population growth rate | May 03, 2019 08:36 |
| 1-phenyl 2-thiourea | May 03, 2019 08:37 |
Abstract
This AOP is designed to estimate changes in population trajectory of fishes resulting from the inhibition of the enzyme tyrosinase (TYR), which is rate-limiting in the control of melanogenesis. Since tyrosinase inhibition leads to the decrease of DOPAquinone synthesis, tyrosinase inhibition by unknown or known chemicals will lead to L DOPA quinone inhibition and decrease of eu - and pheo -melanogenesis. Subsequently, these KEs possibly lead to the decline of teleost population. Hence this AOP could support the use of an in vitro high throughput screening assay for tyrosinase inhibition to identify chemicals that may reduce pigmentation in fish leaving them vulnerable to predation and unable to perform important social behaviors important to their survival and reproduction. Decreased population trajectory resulting from reduced pigmentation patterns in the fish body is a potential endpoint for eco-toxicity. The proposed endpoint will provide useful high throughput risk assessment screening tools for potential chemicals. Consequently, this AOP can be applied to the prediction of eco-toxicity caused by the inhibition of TYR.
AOP Development Strategy
Context
|
The present AOP shows a tyrosinase (TYR) inhibition-mediated adverse outcome (AO) in fishes. TYR is the rate-limiting enzyme controlling the induction of melanogenesis in diverse colored patterns in aquatic organisms. The significant reactions of TYR can be considered that the tyrosinase inhibitor-induced depigmentation reduces the trajectory of fishes. Acknowledgements: This research was supported by the National Research Council of Science & Technology(NST) grant by the Korea government (MSIP) (No. CAP-17-01-KIST Europe) |
Strategy
Summary of the AOP
Events:
Molecular Initiating Events (MIE)
Key Events (KE)
Adverse Outcomes (AO)
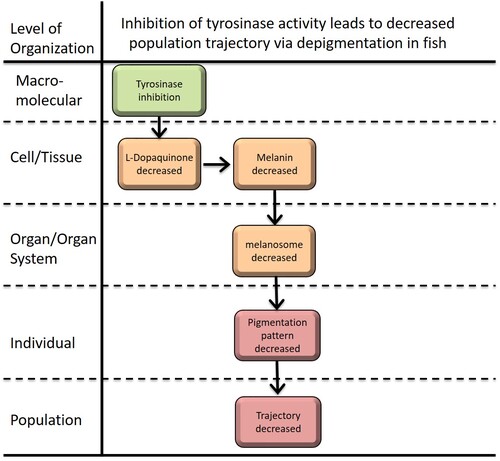
| Type | Event ID | Title | Short name |
|---|
| MIE | 1627 | Inhibition of tyrosinase | tyrosinase |
| KE | 1628 | Reduction of L-Dopaquinone | Reduction of L-Dopaquinone |
| KE | 1629 | Reduction in melanin level | Reduction in melanin level |
| KE | 1630 | Reduction of melanosome level | Reduction of melanosome level |
| KE | 1631 | Reduction fo Pigmentation pattern | Reduction fo Pigmentation pattern |
| AO | 360 | Decrease, Population growth rate | Decrease, Population growth rate |
Relationships Between Two Key Events (Including MIEs and AOs)
| Title | Adjacency | Evidence | Quantitative Understanding |
|---|
| tyrosinase leads to Reduction of L-Dopaquinone | adjacent | High | High |
| Reduction of L-Dopaquinone leads to Reduction in melanin level | adjacent | High | High |
| Reduction in melanin level leads to Reduction of melanosome level | adjacent | High | High |
| Reduction of melanosome level leads to Reduction fo Pigmentation pattern | adjacent | High | High |
| Reduction fo Pigmentation pattern leads to Decrease, Population growth rate | adjacent | Moderate | Low |
Network View
Prototypical Stressors
| Name |
|---|
| 1-phenyl 2-thiourea |
Life Stage Applicability
| Life stage | Evidence |
|---|---|
| Not Otherwise Specified |
Taxonomic Applicability
| Term | Scientific Term | Evidence | Link |
|---|---|---|---|
| fish | fish | High | NCBI |
Sex Applicability
| Sex | Evidence |
|---|---|
| Mixed | Moderate |
Overall Assessment of the AOP
|
To do |
|
|
Building the AOP frame |
Development of KEs |
|
Production of experimental data |
|
|
Overall assessment of the AOP |
Biological domain of applicability |
|
Essentiality of all KEs |
|
|
Evidence supporting all KERs |
|
|
Quantitative WoE considerations |
|
|
Quantitative understanding for each KER |
|
Domain of Applicability
Essentiality of the Key Events
Inhibition of TYR (KER 1627) can be caused by chemical inhibitors such as 1-phenyl 2-thiourea (PTU), sesamol, arbutin, Kojic acid, bis(4-hydroxybenzyl) and etc. (J. Karlsson et al., 2001; W. C. Chen et al., 2015; Baek and Lee, 2015; S. H. Cha et al., 2011).
TYR inhibition as Key event 1891, the MIE for the present AOP, results in reduction of L-Dopaquinone level in the melanocyte via inhibition of L-DOPA oxidation moreover, it results in attenuation of eumelanin and pheomelanin biosynthesis (T. S. Chang, 2012; J. Choi and J. G. Jee, 2015; S. Y. Lee, 2016; A. J. Winder and H. Harris, 1991; W. C. Chen et al., 2015).
There are perhaps a non-adjacent relationship linking event 1629 to event 1631 that the lowered level of melanin biosynthesis by TYR inhibition simultaneously leads to depigmentation in skin tissue and diminished pigmentation pattern in the fish body (L. E. Jao et al., 2013; S. Y. Wu et al., 2015; S. H. Baek and S. H. Lee, 2015; W. C. Chen et al., 2015; D. C. Kim et al., 2017).
Evidence Assessment
First, TYR can convert L-tyrosine directly to L-3,4-dihydroxyphenylalanine (L-DOPA) which is a precursor of (2S)-2-Amino-3-(3,4-dioxocyclohexa-1,5-dien-1-yl)propanoic acid (L-Dopaquinone) synthesis; Second, TYR catalyzes the oxidation of L-DOPA to the L-Dopaquinone which is the reactive intermediate for the eumelanin and pheomelanin synthesis. Pigment patterns in common fishes usually play a significant role to communicate within species, intersexual interactions, escape potential in the eyes of predators and finding shoal mate (Price et al., 2008; C. L. Peichel et al., 2004; R. E. Engeszer et al., 2004)
Known Modulating Factors
Quantitative Understanding
It will be come soon,
Considerations for Potential Applications of the AOP (optional)
In fish as behavioral ecology, color patterns are often multi-component signals, composed of pigment-based and physiological regulation that can be used to communicate in both inter- and intrasexual interactions in population. This endpoint is essential and useful for screening of pigmentation effects on the photosensitive context for skin toxicity screening and relevant to teratogenic effects.
References
References
Karlsson, J., et al. (2001). Generating transparent zebrafish: a refined method to improve detection of gene expression during embryonic development. Marine Biotechnology, 3(6), 522-527.
Chen, W. C., et al. (2015). Discovery of highly potent tyrosinase inhibitor, T1, with significant anti-melanogenesis ability by zebrafish in vivo assay and computational molecular modeling. Scientific reports, 5, 7995.
Baek, S. H., & Lee, S. H. (2015). Sesamol decreases melanin biosynthesis in melanocyte cells and zebrafish: Possible involvement of MITF via the intracellular cAMP and p38/JNK signalling pathways. Experimental dermatology, 24(10), 761-766.
Cha, S. H., et al. (2011). Screening of marine algae for potential tyrosinase inhibitor: those inhibitors reduced tyrosinase activity and melanin synthesis in zebrafish. The Journal of dermatology, 38(4), 354-363.
Chang, T. S. (2012). Natural melanogenesis inhibitors acting through the down-regulation of tyrosinase activity. Materials, 5(9), 1661-1685.
Choi, J., & Jee, J. G. (2015). Repositioning of thiourea-containing drugs as tyrosinase inhibitors. International journal of molecular sciences, 16(12), 28534-28548.
Lee, S. Y., Baek, N., & Nam, T. G. (2016). Natural, semisynthetic and synthetic tyrosinase inhibitors. Journal of enzyme inhibition and medicinal chemistry, 31(1), 1-13.
Winder, A. J., & Harris, H. (1991). New assays for the tyrosine hydroxylase and dopa oxidase activities of tyrosinase. European journal of biochemistry, 198(2), 317-326.
Chen, W. C., et al. (2015). Discovery highly potent tyrosinase inhibitor, T1, with significant anti-melanogenesis ability by zebrafish in vivo assay and computational molecular modeling. Scientific reports, 5, 7995.
Jao, L. E., et al. (2013). Efficient multiplex biallelic zebrafish genome editing using a CRISPR nuclease system. Proceedings of the National Academy of Sciences, 110(34), 13904-13909.
Wu, S. Y., et al. (2015). 4-(Phenylsulfanyl) butan-2-one suppresses melanin synthesis and melanosome maturation in vitro and in vivo. International journal of molecular sciences, 16(9), 20240-20257.
Baek, S. H., & Lee, S. H. (2015). Sesamol decreases melanin biosynthesis in melanocyte cells and zebrafish: Possible involvement of MITF via the intracellular cAMP and p38/JNK signalling pathways. Experimental dermatology, 24(10), 761-766.
Chen, W. C., et al. (2015). Discovery of highly potent tyrosinase inhibitor, T1, with significant anti-melanogenesis ability by zebrafish in vivo assay and computational molecular modeling. Scientific reports, 5, 7995.
Kim, D. C., et al. (2017). p-coumaric acid potently down-regulates zebrafish embryo pigmentation: Comparison of in vivo assay and computational molecular modeling with phenylthiourea. Biomedical Science Letters, 23(1), 8-16.
Price, A. C., et al. (2008). Pigments, patterns, and fish behavior. Zebrafish, 5(4), 297-307.
Peichel, C. L. (2004). Social behavior: how do fish find their shoal mate?. Current Biology, 14(13), R503-R504.
Engeszer, R. E., et al. (2004). Learned social preference in zebrafish. Current Biology, 14(10), 881-884.
Slavík, O., et al. (2016). How does agonistic behaviour differ in albino and pigmented fish?. PeerJ, 4, e1937.
Ren, J. Q., et al. (2002). Behavioral visual responses of wild-type and hypopigmented zebrafish. Vision research, 42(3), 293-299.
Slavík, O., et al. (2015). Ostracism of an albino individual by a group of pigmented catfish. Plos one, 10(5), e0128279.
Onyia, U. L., et al. (2016). Growth and survival of normal coloured and albino clarias gariepinus and their reciprocal hybrids. Nigerian Journal of Fisheries and Aquaculture, 4(1), 22-27.
Bondari, K. (1984). Comparative performance of albino and normally pigmented channel catfish in tanks, cages, and ponds. Aquaculture, 37(4), 293-301.
Pérez-Carpinell, J. O. A. Q. U. I. N., et al. (1992). Vision defects in albinism. Optometry and vision science: official publication of the American Academy of Optometry, 69(8), 623-628.
Cho, K., Ryu, C. S., Jeong, S., & Kim, Y. (2020). Potential adverse effect of tyrosinase inhibitors on teleosts: A review. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology, 228, 108655.
Maki JA, Cavallin JE, Lott KG, Saari TW, Ankley GT, Villeneuve DL. A method for CRISPR/Cas9 mutation of genes in fathead minnow (Pimephales promelas). Aquat Toxicol. 2020;222:105464. doi:10.1016/j.aquatox.2020.105464