
This AOP is licensed under the BY-SA license. This license allows reusers to distribute, remix, adapt, and build upon the material in any medium or format, so long as attribution is given to the creator. The license allows for commercial use. If you remix, adapt, or build upon the material, you must license the modified material under identical terms.
AOP: 313
Title
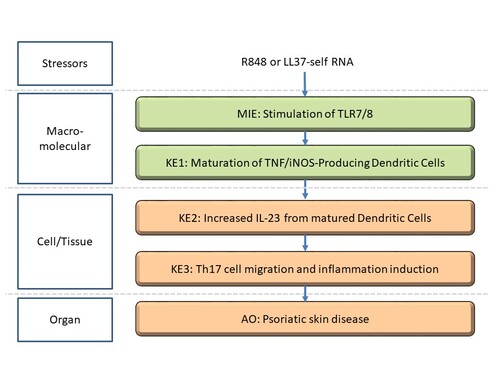
Stimulation of TLR7/8 in dendric cells leading to Psoriatic skin disease
Short name
Graphical Representation
Point of Contact
Contributors
- Takumi Ohishi
- Hiroyuki Komatsu
Coaches
- Julija Filipovska
OECD Information Table
| OECD Project # | OECD Status | Reviewer's Reports | Journal-format Article | OECD iLibrary Published Version |
|---|---|---|---|---|
| 1.75 | Under Development |
This AOP was last modified on April 29, 2023 16:03
Revision dates for related pages
| Page | Revision Date/Time |
|---|---|
| Stimulation, TLR7/8 | November 21, 2020 07:14 |
| Increase, IL-23 from matured dendritic cells | November 23, 2020 06:44 |
| Th17 cell migration and inflammation induction | November 23, 2020 06:59 |
| Psoriatic skin disease | November 23, 2020 07:04 |
| Maturation of TNF/iNOS-Producing Dendritic Cells | November 23, 2020 06:38 |
| Stimulation of TLR7/8 leads to Increase of IL-23 | December 27, 2019 05:33 |
| Increase of IL-23 leads to Th17 cell migration and inflammation induction | December 27, 2019 05:35 |
| Th17 cell migration and inflammation induction leads to Skin disease | December 27, 2019 05:41 |
| Imiquimod | December 27, 2019 04:57 |
| Resiquimod | December 27, 2019 04:58 |
Abstract
Toll-like receptor (TLR) 7 and TLR8 are pattern recognition receptors that are known to activate antiviral reaction of immune system, hyperactivation of which can lead to psoriatic skin disease when hyperactivation of them occurred. The relationship between TLR7/8 and immune functions is well understood, and antiviral compound that work by stimulating TLR7/8 have been developed. TLR7/8 agonists such as imidazoquinolin compounds stimulate these TLRs through the formation of homodimer. This signal activates the IL-23/IL-17 axis, which leads to psoriasis and other related skin diseases.
Activation of the IL-23 / IL-17 axis and causes abnormal proliferation and inflammation of the epidermis, which is a pathological condition of psoriasis. This AOP shows an association between TLR7 / 8 stimulation and psoriatic skin disease.
TLR7-mediated signaling in plasmacytoid dendric cells (pDC) is mediated in a MyD88-dependent fashion, which initiates an IRF7, IRAK1, TRAF6, TRAF3, and IKKα-mediated response, secreting vast amounts of IFN type 1. Similarly, upon engagement of ligands in endosomes, TLR8 initiate the MyD88-dependent pathway culminating in synthesis and release of proinflammatory mediators, such as TNF-α via NF-κB activation. IFN-α and TNF-α cooperatively mature myeloid dendritic cells. TLR7/8 agonist stimulates a specific population of inflammatory dermal dendritic cells referred as TNF and inducible nitric oxide synthase–expressing DCs (Tip-DCs) to produce IL-23 after maturation by enhanced transcriptional activity.
IL-23R is mainly expressed in Th17 cells. In chronic psoriasis, the cytokines IL-12 and IL-23 produced by resident DC are the main causes. Not only does the expression of IL-23 increases in the skin tissue of the lesion, Th17 cells also increase.
Mature Th17 cells are activated by IL-23 stimulation. Signaling through IL-23 produces cytokines IL-17 and IL-22 that mediate the psoriasis response and promote neutrophil migration into the epidermis, epidermal cell proliferation, and similar responses, which lead to the development of a psoriasis rash. In mice, psoriasis-like hyperplasia is induced by the application of IL-23 but does not occur in IL-17A and IL-22 KO mice, so IL-17A and IL-22 play an important role downstream of IL-23.
IL-17 receptor form heterodimers, and IL-17RA / IL-17RC appears in a variety of cells, including fibroblasts and epidermal cells. IL-17RE / IL-17RA expressed in epidermal cells and IL-17C binding are also important in the pathology of psoriasis. Immunohistochemically, IL-17A is expressed only in cells of the dermal papilla layer, while IL-17C is widely expressed in cells such as hyperproliferative overexpressed keratinocytes, leukocytes, and vascular endothelial cells. IL-17C produces keratinocytes by bacterial stimulation and further stimulates keratinocytes to induce the production of various cytokines and chemokines. Keratinocytes are known to be self-activated by IL-17C.
IL-17 and IL-22 secreted from Th17 act on keratinocytes, causing abnormalities in keratinocytes through the secretion of inflammatory cytokines, chemokines, growth factors, and antimicrobial peptides, and thereby exacerbating the skin symptoms of psoriasis.
The creation of this AOP began with an examination of important event relationships brought about by TLR7 / 8 activity due to environmental or genetic factors and resulting in abnormal differentiation of keratinocytes, which leads to thickening of the epidermis and its resultant autoimmune skin disease, psoriasis
AOP Development Strategy
Context
Psoriasis is an chronic autoimmune disease characterized by chronic epithelial inflammatory disease induced by environmental factors such as infection, stress, smoking or alcohol consumption as well as by genetic factors. The onset of psoriasis has been reported to be triggered by drugs and chemical substances use, including beta-blockers, chloroquine, lithium, ACE inhibitors, indomethacin, terbinafine, and interferon alpha. Diagnosis is based on the type and distribution of the lesions.
Psoriasis occurs when abnormal differentiation (keratosis) of keratinocytes leads to thickening of the epidermis. Patients often exhibit an erythema with a clear border and epidermal hyperplasia, stratum corneum hyperplasia, heterocytosis in the stratum corneum, mixed skin moist cells of neutrophilic granulocytes and T cells in the epidermis. Dendritic cells (DC) and macrophages are associated with silver-white plaque. Neutrophilic effusion (Munro microabscesses) are observed in the epidermis, and CD8+ T cells (Tc17) increase the expression of angiogenesis related genes.
The main therapeutic agents are mild topical treatments such as emollients, salicylic acid, coal tar preparations, anthralin, corticosteroids, vitamin D3 derivatives, retinoids, calcineurin inhibitors or tazarotene. UV therapy is also used for moderate or severe psoriasis. Widespread psoriasis is treated with systemic therapies such as immunomodulators methotrexate, cyclosporin, retinoids and other immunosuppressants used alone or in combination.
Although there are stressors that are well known to induce psoriasis-like skin inflammation in mice, this AOP is based primarily on an understanding of stimulation caused by imiquimod, resiquimod or LL37-selfRNA complexes, for which a significant body of scientific literature has been published.
As a test model for psoriasis, an Autoimmune skin disease, mouse tests that induce skin inflammation like psoriasis are frequently conducted using the imidazoquinoline derivative imiquimod. This AOP is primarily based on an understanding of stimuli caused by imiquimod, resiquimod, or LL37-selfRNA complexes.
Imiquimod is derived from imidazoquinoline and is often used to create mouse models. It is our hope that this AOP will contribute to greater knowledge about the development of psoriatic skin diseases that start from stimulation of TLR as well as the development of new treatment targets for psoriasis.
Strategy
Summary of the AOP
Events:
Molecular Initiating Events (MIE)
Key Events (KE)
Adverse Outcomes (AO)
| Type | Event ID | Title | Short name |
|---|
| MIE | 1706 | Stimulation, TLR7/8 | Stimulation of TLR7/8 |
| KE | 1822 | Maturation of TNF/iNOS-Producing Dendritic Cells | Maturation, TNF/iNOS-Producing Dendritic Cells |
| KE | 1707 | Increase, IL-23 from matured dendritic cells | Increase of IL-23 |
| KE | 1708 | Th17 cell migration and inflammation induction | Th17 cell migration and inflammation induction |
| AO | 1709 | Psoriatic skin disease | Skin disease |
Relationships Between Two Key Events (Including MIEs and AOs)
| Title | Adjacency | Evidence | Quantitative Understanding |
|---|
| Stimulation of TLR7/8 leads to Increase of IL-23 | adjacent | High | High |
| Increase of IL-23 leads to Th17 cell migration and inflammation induction | adjacent | High | High |
| Th17 cell migration and inflammation induction leads to Skin disease | adjacent | High | High |
Network View
Prototypical Stressors
| Name |
|---|
| Imiquimod |
| Resiquimod |
Life Stage Applicability
| Life stage | Evidence |
|---|---|
| All life stages | Not Specified |
Taxonomic Applicability
Sex Applicability
| Sex | Evidence |
|---|---|
| Mixed | High |
Overall Assessment of the AOP
TLR7/8 is stimulated when imidazoquinolin compounds or stimilar agonists from homodimers TLR7-mediated signaling in plasmacytoid dendritic cells (pDC) is mediated in a MyD88-dependent fashion, which initiates an IRF7, IRAK1, TRAF6, TRAF3, and IKKα-mediated response,thereby secreting large amounts of IFN-α. Similarly, the engagement of ligands in endosomescauses TLR8 initiate the MyD88-dependent pathway, culminating in synthesis and release of TNF-a and other proinflammatory mediators, via NF-κB activation.
IFN-α and TNF-α cooperatively mature myeloid dendritic cells. TLR7/8 agonist stimulates a specific population of inflammatory dermal dendritic cells referred as Tip-DCs to produce IL-23 after maturation by enhanced transcriptional activity.
Naive T cells differentiate into Naive Th17 by both IL-6 and TGF-β cells that express the transcription factors ROR-γt, ROR-α, and STAT3. These naive Th17 cells are self-activated by IL-21 in an autocrine manner and mature into Th17 cells which express IL-23 receptor on cell surface. Mature Th17 cells are activated by IL-23 stimulation. IL-23-mediated signal transduction produces cytokines IL-17.
IL-17 mediates the psoriasis response, promoting such activities as neutrophil migration to the epidermis,and proliferation of epidermal cells, which leads to the outbreak of psoriasis rash. Thus, psoriatic skin is induced mainly by overproduction of IL-17, which leads to a variety of adverse effects. We have identified a number of key events (KEs) along this pathway and created an AOP for stimulation of TLR7/8 that leads to psoriatic skin disease based on these key event relationships(KERs).
Domain of Applicability
The proposed AOP for psoriasis-like skin thickening resulting from abnormal differentiation of keratinocytes, starting with Toll-like receptor (TLR) 7/8 activity, is independent of life stage, gender, or age (Lowes et al. 2007). The pathogenesis of psoriasis, an autoimmune disease, is genetically predisposed (3), but the autoantigen that causes psoriasis has not been identified (Zaba et al. 2008). Other causes of psoriasis are caused by external and internal triggers such as mild trauma, sunburn, infection, systemic drugs, and stress (Hansel et al. 2011). Stimulation of TLR7 / 8 releases INF-α and TNF-α in large amounts to produce IL-23, and Th17 cells mature by the stimulation to produce IL-17 and IL-22. In psoriasis skin formation, cytokines such as TNF-α, IL-23, and IL-17 work continuously. Since TNF-α inhibitors significantly suppressed IL-17A and IL-23p19 expression in psoriatic eruptions (Leonardi et al. 2012), by suppressing self-activation of Tip-DC by TNF-α, It can be seen that IL-23 and IL-17A production was suppressed. Anti-IL-17 and anti-IL-17RA antibodies suppress IL-17A and IL-17C, which are highly expressed in psoriatic eruptions. In particular, anti-IL-17RA antibody has been shown to normalize the expression of keratinocyte-related genes and IL-17C production two weeks after administration, followed by normalization of IL-17A production from leukocytes.
In mice, subcutaneous administration of IL-23 induced psoriatic eruption and IL-17A expression (K. A. et al. 2013), and IL-17C transgenic mice overexpressing IL-17C in keratinocytes showed psoriatic eruption. As shown in (8), the reaction of psoriasis-like eruption occurs in mice due to the chain of stimulation to T cells and epidermal cells starting from TLR.
Essentiality of the Key Events
Stressor, MIE and later events:MyD88 knock out(KO) mice
TLR7 (TLR7 / 8 in human) recognizes the imidazoquinoline derivative, binds to the adapter molecule MyD88, activates IRAKs (IL-1 receptor associated kinases), interacts with TRAF6 (TNF receptor associated factor 6) and IKK (Activates the IκB kinase complex). It phosphorylates IκB, induces its degradation, and transfers the transcription factor NF-κB to the nucleus. This pathway is called MyD88-dependent pathway and is essential for the production of inflammatory cytokines such as TNF-α (Akira S, Takeda K .: Nat Rev Immunol. Jul; 4: 499-511, 2004). When pDC is stimulated with a TLR7 / 8 ligand, the transcription factor IRF7 constitutively expressing pDC and MyD88 associate directly. IRF7 activity does not occur when pDCs of MyD88 KO mice are stimulated with TLR7 / 8 ligand. IRF7 is also activated by binding to TRAF6, leading to IFN-α production, which requires the Myd88 / TRAF6 / IRF7 complex. (Satoshi U, Shizuo A: Virus 54; 2: 145-152,2004)
Imiquimod 5% cream was applied to the left flank of female SKH-1 hairless mice (25 g body weight). The IFN-α and TNF-α concentrations in the skin after 1 and 2 hours of application increased these concentrations compared to the untreated skin.
In C57BL / 6 mice (8-12 weeks old) sensitized with 0.5% dinitrofluorobenzene (DNFB) as an antigen, imiquimod 5% cream was applied to the auricle once a day for 3 days. The application of imiquimod 5% cream promoted edema of the ears of mice (promoted DTH) compared to the base cream group. Imiquimod activates antigen-specific T cells by topical application to the skin. (Beserna Cream Interview Form Mochida Pharmaceutical Co., Ltd.)
KE-1 and later event:IL-17, IL-22 KO mice
In mice, psoriasis-like hyperplasia is induced by the application of IL-23, but this effect does not occur in IL-17A and IL-22 KO mice. IL-17A deficient mice show little epidermal hyperplasia after intradermal administration of IL-23. WT mice treated with anti-IL-17A Ab did not show IL-23-induced epidermal hyperplasia. IL-17 KO mice treated with IL-23 do not induce TNF-α mRNA and do not cause epidermal thickening. IL-22 did not increase in IL-17-/-mice after IL-23 administration, and IL-17 clearly increased in IL-22-/-mice. In IL-17-/-, IL-22-/-and WT mice treated with IL-23, immunohistochemically CD3 + T cells, CD11c (dendritic cells), F4 / 80 (macrophages), Gr-1 (Neutrophils) were analyzed. There was no difference in F4 / 80 and Gr-1 + cells in IL-17A-/-compared to WT mice, and CD3 + T cells decreased, but there was no obvious difference in IL-22-/-mice .
These data suggest that cytokines alone are not sufficient to mediate IL-23-induced epidermal changes, and that IL-17 and IL-22 are downstream mediators of mouse skin IL-23-induced changes. Therefore, Th17 cytokines are required for the generation of IL-23-mediated skin lesions.
KE-2 and later events: Mouse psoriasis-like dermatitis model
When TPA (12-O-tetradecanoy1phorbol-13-acetate) on the dorsal skin of K14 / mIL-1F6 gene-modified mice overexpress mouse IL-1F6 (IL-36a) selectively under the keratin 14 promoter was applied, skin pathological findings specific to psoriasis were observed, such as epidermal hyperplasia, epidermal exfoliation and micro-abscess formation, and wet inflammatory cells in the dermis. Quantitative RT-PCR measures mRNA expression levels of inflammatory chemokines and cytokines in skin tissues, and includes inflammatory chemokines: CCL3, CCL4, CXCL10, CXCL1, and cytokines: IL-23, IL-12, IL-1β, etc. These expressions were elevated. (Kyowa Hakko Kirin Co., Ltd.)