
The authors have designated this AOP as all rights reserved. Re-use in any form requires advanced permission from the authors.
AOP: 491
Title
Decrease, GLI1/2 target gene expression leads to orofacial clefting
Short name
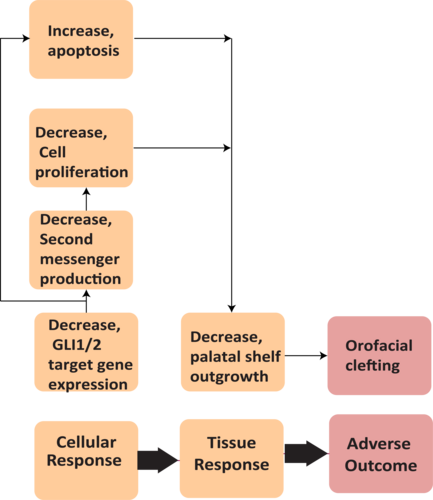
Graphical Representation
Point of Contact
Contributors
- Jacob Reynolds
Coaches
OECD Information Table
| OECD Project # | OECD Status | Reviewer's Reports | Journal-format Article | OECD iLibrary Published Version |
|---|---|---|---|---|
| 1.101 | Under Development |
This AOP was last modified on October 16, 2023 11:17
Revision dates for related pages
| Page | Revision Date/Time |
|---|---|
| Decrease, GLI1/2 target gene expression | April 02, 2025 11:24 |
| Decrease, Sonic Hedgehog second messenger production | March 22, 2023 12:09 |
| Decrease, Cell proliferation | December 07, 2020 06:55 |
| Decrease, facial prominence outgrowth | April 03, 2025 13:05 |
| Increase, Orofacial clefting | April 03, 2025 13:10 |
| Apoptosis | May 31, 2025 08:50 |
| Decrease, GLI1/2 target gene expression leads to Decrease, SHH second messenger production | April 03, 2025 11:56 |
| Decrease, SHH second messenger production leads to Decrease, Cell proliferation | April 03, 2025 13:03 |
| Decrease, Cell proliferation leads to Decrease, facial prominence outgrowth | April 02, 2025 14:03 |
| Decrease, SHH second messenger production leads to Apoptosis | June 29, 2023 13:41 |
| Decrease, facial prominence outgrowth leads to orofacial cleft | May 22, 2023 12:36 |
| Apoptosis leads to Decrease, facial prominence outgrowth | April 04, 2025 10:48 |
| GANT 58 | October 18, 2022 11:42 |
| GANT61 | October 18, 2022 11:42 |
Abstract
This Adverse Outcome Pathway (AOP) describes the linkage between antagonism of the Smoothened (SMO) receptor and orofacial clefts (OFCs) (AOP 460 in the Collaborative Adverse Outcome Pathway Wiki). Sonic Hedgehog (SHH) is a major signaling pathway of intercellular signaling during embryogenesis including the morphogenesis of the face. The SHH pathway is sensitive to chemical induced disruption at multiple points including through disruption to GLI1/2 target gene expression. Activation of the SHH pathway causes a signaling cascade that culminates with the transcription of GLI transcription factors and can occur in an autocrine, paracrine, or juxtracrine manner. When GLI1/2 target gene expression is disrupted during critical windows of development, this signaling cascade is disrupted resulting in reduced outgrowth of the facial prominences and the formation of an OFC. While most of the adjacent events lack studies showing dose or time response for the relationships, there is a high biological plausibility of the proposed AOP. We performed literature searches using Pubmed with Medical Subject Headings (MeSH) terms associated with the key events (KE) of interest. Sources were initially screened for relevance through review of the title and abstract. Those selected were reviewed to determine what if any data existed to support or refute the key event relationship (KER). While this AOP is specific to mouse (mus musculus) during embryonic development, SHH and the development of the face is largely conserved between mouse and human making this AOP able to be extrapolated to risk assessment for human exposures. It is hoped that further studies will be performed to increase the weight of evidence (WoE) for this pathway. This AOP is intended to serve as a tool for risk assessment for drug and chemical exposures during embryonic development when disruption to SHH through a decrease in GLI1/2 target gene expression occurs.
AOP Development Strategy
Context
Orofacial clefts (OFCs), encompassing cleft lip with or without palate (CL/P), and cleft palate only (CPO) represent the second most common birth defect in humans with a prevalence of 1-2/1,000 births (Lidral, Moreno et al. 2008). The etiology of OFCs is complex with approximately 50% of CPO and 70% of CL/P considered non-syndromic (2011). SHH signaling is required for normal facial development and plays a critical role in the growth of the facial processes that form the upper palate and lip (Bush and Jiang 2012, Kurosaka 2015). The epithelial derived SHH drives orofacial development through an induced gradient in the underlying mesenchyme (Lan and Jiang 2009, Kurosaka 2015). This gradient of SHH induces cellular proliferation and outgrowth of the mesenchyme (Lan and Jiang 2009). The SHH pathway is sensitive to chemical disruption and can be disrupted at multiple places along the signaling cascade during critical windows for exposure and has been shown to cause OFCs (Lipinski and Bushman 2010, Heyne, Melberg et al. 2015). The targets of this disruption include ligand modification, ligand secretion, downstream sensing, and signal transduction (Jeong and McMahon 2002, Lauth, Bergström et al. 2007, Petrova, Rios-Esteves et al. 2013). Chemical modulators of the SHH pathway through antagonism of SMO have been identified including the natural alkaloid cyclopamine, both natural and synthetic pharmaceuticals, and a pesticide synergist (PBO) (Lipinski, Dengler et al. 2007, Lipinski, Song et al. 2010, Wang, Lu et al. 2012, Everson, Sun et al. 2019, Rivera-González, Beames et al. 2021).
Strategy
This AOP was developed as part of a larger network of AOPs linking disruption of SHH signaling with OFCs (OECD Advisory Group on Emerging Science in Chemicals Assessment (ESCA) workplan project 1.101.). Orofacial clefts (OFCs) are one of the most common human birth defects and occur in approximately 1-2/1,000 live births (Lidral, Moreno et al. 2008). Early orofacial development involves epithelial ectoderm derived SHH ligand driving tissue outgrowth through an induced gradient of SHH dependent transcription in the underlying mesenchyme, which is thought to drive mesenchymal proliferation (Lan and Jiang 2009, Kurosaka 2015). The SHH pathway is sensitive to chemical disruption at multiple molecular targets along the signaling cascade, with exposure during critical windows in development leading to OFCs (Lipinski and Bushman 2010, Heyne, Melberg et al. 2015). The molecular targets of this disruption include SHH ligand modification with cholesterol and palmitoylate, ligand secretion, mesenchymal reception, and signal transduction (Jeong and McMahon 2002, Lauth, Bergström et al. 2007, Petrova, Rios-Esteves et al. 2013). This AOP focuses on the disruption to SHH signaling through a decrease in GLI1/2 target gene expression. To select the key events for the AOP, we used existing knowledge of the pathway along with reviews of the SHH pathway to assemble a path that was physiologically plausible. Care was taken to select events that would be of direct regulatory relevance (i.e. a method to quantify exists). To identify sources and data for each Key Event Relationship (KER), Pubmed was used. Initially results were screened for relevance off title/abstract and any of suspected relevance were reviewed in full to determine their applicability for the KER. Each KER includes a table of relevant search information (date, search terms, citations, etc). It is the hope of the authors that this AOP is used as a tool for risk assessment for drug and chemical exposures during embryonic development when disruption to SHH through decreased GLI1/2 gene expression occurs.
Summary of the AOP
Events:
Molecular Initiating Events (MIE)
Key Events (KE)
Adverse Outcomes (AO)
| Type | Event ID | Title | Short name |
|---|
| MIE | 2040 | Decrease, GLI1/2 target gene expression | Decrease, GLI1/2 target gene expression |
| KE | 2043 | Decrease, Sonic Hedgehog second messenger production | Decrease, SHH second messenger production |
| KE | 1821 | Decrease, Cell proliferation | Decrease, Cell proliferation |
| KE | 2041 | Decrease, facial prominence outgrowth | Decrease, facial prominence outgrowth |
| KE | 1262 | Apoptosis | Apoptosis |
| AO | 2042 | Increase, Orofacial clefting | orofacial cleft |
Relationships Between Two Key Events (Including MIEs and AOs)
| Title | Adjacency | Evidence | Quantitative Understanding |
|---|
| Decrease, GLI1/2 target gene expression leads to Decrease, SHH second messenger production | adjacent | Low | Low |
| Decrease, SHH second messenger production leads to Decrease, Cell proliferation | adjacent | Low | Low |
| Decrease, Cell proliferation leads to Decrease, facial prominence outgrowth | adjacent | Low | Low |
| Decrease, SHH second messenger production leads to Apoptosis | adjacent | Low | Low |
| Decrease, facial prominence outgrowth leads to orofacial cleft | adjacent | Moderate | Low |
| Apoptosis leads to Decrease, facial prominence outgrowth | adjacent | Low | Low |
Network View
Prototypical Stressors
Life Stage Applicability
| Life stage | Evidence |
|---|---|
| Embryo | High |
Taxonomic Applicability
| Term | Scientific Term | Evidence | Link |
|---|---|---|---|
| mouse | Mus musculus | NCBI |
Sex Applicability
| Sex | Evidence |
|---|---|
| Unspecific |
Overall Assessment of the AOP
Domain of Applicability
weight of evidence elements is attached in PDF format.
Domain(s) of Applicability
Chemical: This AOP applies to compounds that decrease GLI1/2 gene expression. Examples include GANT 58 and GANT 61.
Sex: This AOP is unspecific to sex.
Life Stages: The relevant life stage for this AOP is embryonic development. More specifically, the development of the craniofacial region which occurs between GD 10.0 and GD 14.0 in the mouse and week 4-12 in human.
Taxonomic: At present, the assumed taxonomic applicability domain of this AOP is mouse (mus musculus). Most of the toxicological data that this AOP is based on has used mice as their model. Mice are a good analog of human craniofacial development and undergo similar signaling by SHH.
Essentiality of the Key Events
Essentiality of the Key Events
To date, few studies have addressed the essentiality of the proposed sequence of key events. Evidence linking SHH disruption through a decrease in proliferation exists. The hypothesized sequence of events has a high temporal concordance for canonical SHH signaling pathway and orofacial development.
- Studies have shown that SHH signaling is required for normal facial development and plays a critical role in the growth of the facial processes that form the upper palate and lip (Bush and Jiang 2012, Kurosaka 2015).
- The epithelial derived SHH drives orofacial development through an induced gradient in the underlying mesenchyme (Lan and Jiang 2009, Kurosaka 2015). This gradient of SHH induces cellular proliferation and outgrowth of the mesenchyme (Lan and Jiang 2009).
- OFCs caused by disruption to SHH are believed to be due to a reduction in epithelial induced proliferation and the subsequent decrease in tissue outgrowth and the failure of the facial processes to meet and fuse (Lipinski, Song et al. 2010, Heyne, Melberg et al. 2015).
Evidence Assessment
Evidence Assessment
- KER ID-Title-[Adjacency], [Evidence], [Quantitative Understanding]
- KER 2731-Decrease, GLI1/2 target gene expression leads to Decrease, SHH second messenger production-[Adjacent], [Low], [Low]-Coordinated signaling is paramount for proper embryonic development and the GLI signaling cascade drives feedback/forward loops with FGF and BMP signaling pathways. Support was found for SHH having a feedforward loop with FGF10 and BMP4 however further investigation into the interaction of these pathways and their crosstalk is required.
- KER 2732-Decrease, SHH second messenger production leads to Decrease, Cell proliferation-[Adjacent], [Low], [Low]- SHH is a known mitogen and drives proliferation through its’ secondary messengers. SHH was found to induce proliferation and FGF10 in vivo. In FGF10 deficient models SHH was found to be reduced.
- KER 2724-Decrease, Cell proliferation leads to Decrease, outgrowth-[Adjacent], [Low], [Low]-SHH is a known mitogen that helps to drive the proper development of the face which includes the outgrowth of the facial prominences. To date, few studies have measured by outgrowth of the facial prominences and proliferation. Hypoplasia of pharyngeal arch 1 was found in SHH-/- embryos supporting that outgrowth is driven by proliferation and is reduced when proliferation is decreased.
- KER 2726-Decrease, outgrowth leads to OFC-[Adjacent], [Moderate], [Low]- OFCs caused by disruption to SHH are believed to be due to a reduction in epithelial induced proliferation and the subsequent decrease in tissue outgrowth and the failure of the facial processes to meet and fuse (Lipinski, Song et al. 2010, Heyne, Melberg et al. 2015). Mice with disrupted SHH signaling are found to have palatal shelves that are spaced apart supporting that the cleft results from an EMi dependent, but epithelial-mesenchyme transition (Emt) independent manner.
- KER 2792-Apoptosis leads to Decrease, Outgrowth-[Adjacent], [Low], [Low]- SHH signaling is known to be associated with cell survival and there is a high biological plausibility that increasing apoptosis would cause a decrease in outgrowth. Supporting evidence is offered with increases in apoptosis in the mandibular arch seen in SHH signaling disrupted mice that exhibit decreased outgrowth.
- KER 2882-Decrease, GLI1/2 target gene expression leads to Apoptosis-[Adjacent], [Low], [Low]- To date few studies have examined the relationship of GLI1/2 target gene expression. There is a high biological plausibility that SHH plays a role in cell survival and death through GLI1/2 target gene expression. Decreased GLI1/2 target gene expression is seen in RA exposed dams alongside increased apoptosis on the cranial neural crest cells (CNCC).
Biological Plausibility Biological plausibility refers to the structural and/or functional relationship that exists between the key events based on our understanding of normal biology. SHH signaling is largely conserved in mammals and is required for normal facial development and plays a critical role in the growth of the facial processes that form the upper palate and lip (Bush and Jiang 2012, Kurosaka 2015).
Concordance of dose-response relationships
There are a limited number of studies in which multiple key events were assessed in the same study following exposure to known SMO antagonists. These studies form the basis of the dose-response concordance of this AOP. A summary of the dose-concordance can be found in table 1. Many studies were found to use a single exposure.
Temporal concordance
Temporal concordance refers to the degree to which the data supports the hypothesized sequence of Molecular Initiating Event (MIE) leading to the Adverse Outcome (AO) through a series of Key Events (KEs). The SHH pathway is a well-known developmental pathway that plays a role in embryogenesis including the development of the face. The SHH pathway is sensitive to chemical disruption at multiple molecular targets along the signaling cascade including antagonism of the SMO receptor, with exposure during critical windows in development leading to OFCs (Lipinski and Bushman 2010, Heyne, Melberg et al. 2015). Chemical modulators of the SHH pathway have been identified including the natural alkaloid cyclopamine, both natural and synthetic pharmaceuticals, and a widely used pesticide synergist (PBO) with established human exposures (Lipinski, Dengler et al. 2007, Lipinski, Song et al. 2010, Wang, Lu et al. 2012, Everson, Sun et al. 2019, Rivera-González, Beames et al. 2021). Canonical SHH signaling through PTCH-SMO-GLI is well understood and our AOP remains consistent with the pathway. SHH signaling is required for normal facial development and plays a critical role in the growth of the facial processes that form the upper palate and lip (Bush and Jiang 2012, Kurosaka 2015). The epithelial derived SHH drives orofacial development through an induced gradient in the underlying mesenchyme (Lan and Jiang 2009, Kurosaka 2015). This gradient of SHH induces cellular proliferation and outgrowth of the mesenchyme (Lan and Jiang 2009). The hypothesized sequence of events is supported by the existing data and follow the field’s current understanding of the canonical SHH signaling pathway.
Consistency
The AO is not specific to this AOP. Many of the events is this AOP will overlap with AOPs linking disruption of SHH to OFC and some are expected to overlap with AOPs linking other developmental signaling pathways to OFCs.
Uncertainties, inconsistencies, and data gaps
This AOP would be strengthened by studies examining the dose-response and time-course relationships for these KERs. The main data gaps for this AOP exist in the lack of studies that have examined the relationship in the context of dose response or time course.
Data gaps:
- Dose response and time course studies relating a decrease GLI translocation leads to decrease GLI target gene expression
- Dose response and time course studies relating a Decrease, GLI1/2 target gene expression leads to Decrease, SHH second messenger production
- Dose response and time course studies relating a Decrease, SHH second messenger production leads to Decrease, Cell proliferation
- Dose response and time course studies relating a Decrease, Cell proliferation leads to Decrease, outgrowth
- Dose response and time course studies relating a Decrease, outgrowth leads to OFC
- Dose response and time course studies relating Apoptosis leads to Decrease, Outgrowth
- Dose response and time course studies relating a Decrease, GLI1/2 target gene expression leads to Apoptosis
Inconsistencies:
Uncertainties:
- The relationships and feedback/feedforward loops that exist between SHH and its’ secondary messengers primarily FGF10 and BMP4 are not well understood. More investigation into these relationships is warranted.
- The regulation of proliferation by SHH has been shown but questions to the exact mechanism of regulation remain. Evidence exists that there is likely an intermediate between SHH and regulation of CCND 1 and CCND 2. Some evidence exists that the intermediate could be a member(s) of the FGF family. The relationship between a decrease is SHH secondary messenger production and a decrease in cellular proliferation is plausible and data is shown that supports a decrease in CCND 1 and 2 in correlation with the FGF and SHH pathways. Further studies are needed to further out understanding of the regulation of proliferation by SHH.
- The exact mechanism through which SHH promotes cell survival is not well understood. Further studies are needed to illuminate the mechanism that links SHH signaling with cell survival.
- The relationship between GLI1/2 target gene expression and increased apoptosis has a high biological plausibility although there is currently lack of studies that address this relationship.
Known Modulating Factors
| Modulating Factor (MF) | Influence or Outcome | KER(s) involved |
|---|---|---|
Quantitative Understanding
Assessment of quantitative understanding of the AOP:
The quantitative understanding for this AOP is low. Most of the data found through the literature search was obtained from doses at a single dose and was not conducted with dose-response or time-course in mind. This AOP would benefit from the generation of additional data that addresses these relationships in a dose response and time course methodology to allow for an increased quantitative understanding of the linkage.
Considerations for Potential Applications of the AOP (optional)
Considerations for potential applications of the AOP
The intended use of this AOP from a regulatory standpoint is to improve predictive potential of developmental hazards as they relate to the SHH pathway and OFCs. It is hoped that this AOP can be applied to data from in silico and in vitro high-throughput screening assays (HTS) to guide selection of agents for further investigation in more representative models of orofacial development. Disruption of the Sonic Hedgehog pathway has broader outcomes than just OFCs and SHH is known to play a role in many aspects of embryonic development including patterning of many systems and limb and digit development. This AOP can be used as part of an integrated assessment of toxicity and can help to guide risk assessment for potential exposures during development.
There is a need for development of New Approach Methodologies (NAMs) to increase understanding of the relationships that exist within this AOP to provide facilitate screenings abilities. Humans are exposed to upwards of 80,000 industrial chemicals and natural products, the majority of which have not undergone any type of toxicity testing either alone or in mixtures. Even highly regulated drugs are typically not tested for safety in pregnant women for obvious reasons despite the medical need in this population (Wise 2022). To help address this, we have engineered an in vitro microphysiological model (MPM) model of orofacial development to facilitate the study of both normal and abnormal orofacial development including disruption of SHH (Johnson, Vitek et al. 2021, Reynolds, Vitek et al. 2022). Traditional high throughput screening (HTS) assays are optimized for one pathway: one readout. This oversimplifies toxicant metabolism, intercellular pathway interactions, and ultimately makes the assay not representative of real-life exposures. Problems with HTS in drug discovery have been identified including missing intercellular interactions, co-exposures, and off target safety (Macarron, Banks et al. 2011). We can learn from these identified problems and engineer in vitro systems to more accurately recapitulate the biology to give a more thorough assessment of chemical and drug exposure.
References
(2011). "Prevalence at birth of cleft lip with or without cleft palate: data from the International Perinatal Database of Typical Oral Clefts (IPDTOC)." Cleft Palate Craniofac J 48(1): 66-81.
Bush, J. O. and R. Jiang (2012). "Palatogenesis: morphogenetic and molecular mechanisms of secondary palate development." Development 139(2): 231-243.
Chen, J. K., J. Taipale, M. K. Cooper and P. A. Beachy (2002). "Inhibition of Hedgehog signaling by direct binding of cyclopamine to Smoothened." Genes Dev 16(21): 2743-2748.
Corbit, K. C., P. Aanstad, V. Singla, A. R. Norman, D. Y. R. Stainier and J. F. Reiter (2005). "Vertebrate Smoothened functions at the primary cilium." Nature 437(7061): 1018-1021.
Everson, J. L., M. R. Sun, D. M. Fink, G. W. Heyne, C. G. Melberg, K. F. Nelson, P. Doroodchi, L. J. Colopy, C. M. Ulschmid, A. A. Martin, M. T. McLaughlin and R. J. Lipinski (2019). "Developmental Toxicity Assessment of Piperonyl Butoxide Exposure Targeting Sonic Hedgehog Signaling and Forebrain and Face Morphogenesis in the Mouse: An in Vitro and in Vivo Study." Environ Health Perspect 127(10): 107006.
Goetz, S. C., P. J. Ocbina and K. V. Anderson (2009). "The primary cilium as a Hedgehog signal transduction machine." Methods Cell Biol 94: 199-222.
Heyne, G. W., C. G. Melberg, P. Doroodchi, K. F. Parins, H. W. Kietzman, J. L. Everson, L. J. Ansen-Wilson and R. J. Lipinski (2015). "Definition of critical periods for Hedgehog pathway antagonist-induced holoprosencephaly, cleft lip, and cleft palate." PLoS One 10(3): e0120517.
Incardona, J. P., W. Gaffield, R. P. Kapur and H. Roelink (1998). "The teratogenic Veratrum alkaloid cyclopamine inhibits sonic hedgehog signal transduction." Development 125(18): 3553-3562.
Jeong, J. and A. P. McMahon (2002). "Cholesterol modification of Hedgehog family proteins." The Journal of Clinical Investigation 110(5): 591-596.
Johnson, B. P., R. A. Vitek, M. M. Morgan, D. M. Fink, T. G. Beames, P. G. Geiger, D. J. Beebe and R. J. Lipinski (2021). "A Microphysiological Approach to Evaluate Effectors of Intercellular Hedgehog Signaling in Development." Front Cell Dev Biol 9: 621442.
Kim, J., E. Y. Hsia, A. Brigui, A. Plessis, P. A. Beachy and X. Zheng (2015). "The role of ciliary trafficking in Hedgehog receptor signaling." Sci Signal 8(379): ra55.
Kurosaka, H. (2015). "The Roles of Hedgehog Signaling in Upper Lip Formation." Biomed Res Int 2015: 901041.
Lan, Y. and R. Jiang (2009). "Sonic hedgehog signaling regulates reciprocal epithelial-mesenchymal interactions controlling palatal outgrowth." Development 136(8): 1387-1396.
Lauth, M., A. Bergström, T. Shimokawa and R. Toftgård (2007). "Inhibition of GLI-mediated transcription and tumor cell growth by small-molecule antagonists." Proc Natl Acad Sci U S A 104(20): 8455-8460.
Lidral, A. C., L. M. Moreno and S. A. Bullard (2008). "Genetic Factors and Orofacial Clefting." Semin Orthod 14(2): 103-114.
Lipinski, R. J. and W. Bushman (2010). "Identification of Hedgehog signaling inhibitors with relevant human exposure by small molecule screening." Toxicol In Vitro 24(5): 1404-1409.
Lipinski, R. J., E. Dengler, M. Kiehn, R. E. Peterson and W. Bushman (2007). "Identification and characterization of several dietary alkaloids as weak inhibitors of hedgehog signaling." Toxicol Sci 100(2): 456-463.
Lipinski, R. J., C. Song, K. K. Sulik, J. L. Everson, J. J. Gipp, D. Yan, W. Bushman and I. J. Rowland (2010). "Cleft lip and palate results from Hedgehog signaling antagonism in the mouse: Phenotypic characterization and clinical implications." Birth Defects Res A Clin Mol Teratol 88(4): 232-240.
Lipinski, R. J., C. Song, K. K. Sulik, J. L. Everson, J. J. Gipp, D. Yan, W. Bushman and I. J. Rowland (2010). "Cleft lip and palate results from Hedgehog signaling antagonism in the mouse: Phenotypic characterization and clinical implications." Birth defects research. Part A, Clinical and molecular teratology 88(4): 232-240.
Macarron, R., M. N. Banks, D. Bojanic, D. J. Burns, D. A. Cirovic, T. Garyantes, D. V. Green, R. P. Hertzberg, W. P. Janzen, J. W. Paslay, U. Schopfer and G. S. Sittampalam (2011). Impact of high-throughput screening in biomedical research. Nat Rev Drug Discov. England. 10: 188-195.
Milenkovic, L., M. P. Scott and R. Rohatgi (2009). "Lateral transport of Smoothened from the plasma membrane to the membrane of the cilium." J Cell Biol 187(3): 365-374.
Milenkovic, L., L. E. Weiss, J. Yoon, T. L. Roth, Y. S. Su, S. J. Sahl, M. P. Scott and W. E. Moerner (2015). "Single-molecule imaging of Hedgehog pathway protein Smoothened in primary cilia reveals binding events regulated by Patched1." Proc Natl Acad Sci U S A 112(27): 8320-8325.
Omnell, M. L., F. R. Sim, R. F. Keeler, L. C. Harne and K. S. Brown (1990). "Expression of Veratrum alkaloid teratogenicity in the mouse." Teratology 42(2): 105-119.
Petrova, E., J. Rios-Esteves, O. Ouerfelli, J. F. Glickman and M. D. Resh (2013). "Inhibitors of Hedgehog acyltransferase block Sonic Hedgehog signaling." Nat Chem Biol 9(4): 247-249.
Reynolds, J. I., R. A. Vitek, P. G. Geiger and B. P. Johnson (2022). Engineering Epithelial–Mesenchymal Microtissues to Study Cell–Cell Interactions in Development. Craniofacial Development: Methods and Protocols. S. Dworkin. New York, NY, Springer US: 201-213.
Rivera-González, K. S., T. G. Beames and R. J. Lipinski (2021). "Examining the developmental toxicity of piperonyl butoxide as a Sonic hedgehog pathway inhibitor." Chemosphere 264: N.PAG-N.PAG.
Rohatgi, R., L. Milenkovic, R. B. Corcoran and M. P. Scott (2009). "Hedgehog signal transduction by Smoothened: pharmacologic evidence for a 2-step activation process." Proc Natl Acad Sci U S A 106(9): 3196-3201.
Rohatgi, R., L. Milenkovic and M. P. Scott (2007). "Patched1 regulates hedgehog signaling at the primary cilium." Science 317(5836): 372-376.
Rohatgi, R. and W. J. Snell (2010). "The ciliary membrane." Curr Opin Cell Biol 22(4): 541-546.
Wang, J., J. Lu, R. A. Mook, Jr., M. Zhang, S. Zhao, L. S. Barak, J. H. Freedman, H. K. Lyerly and W. Chen (2012). "The insecticide synergist piperonyl butoxide inhibits hedgehog signaling: assessing chemical risks." Toxicol Sci 128(2): 517-523.
Wang, Y., A. C. Arvanites, L. Davidow, J. Blanchard, K. Lam, J. W. Yoo, S. Coy, L. L. Rubin and A. P. McMahon (2012). "Selective identification of hedgehog pathway antagonists by direct analysis of smoothened ciliary translocation." ACS Chem Biol 7(6): 1040-1048.
Wise, J. (2022). "Pregnant women should be included in clinical trials to improve outcomes, says commission." Bmj 377: o1193.
