
The authors have designated this AOP as all rights reserved. Re-use in any form requires advanced permission from the authors.
AOP: 507
Title
Nrf2 inhibition leading to vascular disrupting effects via inflammation pathway
Short name
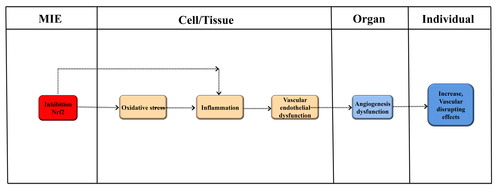
Graphical Representation
Point of Contact
Contributors
- Yanhong Wei
Coaches
OECD Information Table
| OECD Project # | OECD Status | Reviewer's Reports | Journal-format Article | OECD iLibrary Published Version |
|---|---|---|---|---|
This AOP was last modified on July 04, 2024 15:30
Revision dates for related pages
| Page | Revision Date/Time |
|---|---|
| NFE2/Nrf2 repression | June 02, 2017 16:27 |
| Activation of inflammation pathway | May 31, 2022 02:47 |
| increased,Vascular endothelial dysfunction | September 01, 2021 20:37 |
| Increase, Vascular disrupting effects | August 19, 2023 20:12 |
| Angiogenesis dysfunction | August 28, 2023 05:00 |
| Increase, Oxidative Stress | February 11, 2026 07:05 |
| NFE2/Nrf2 repression leads to Activation, inflammation pathway | August 28, 2023 07:47 |
| NFE2/Nrf2 repression leads to Increase, Oxidative Stress | July 04, 2024 15:28 |
| Increase, Oxidative Stress leads to Activation, inflammation pathway | July 04, 2024 15:28 |
| Activation, inflammation pathway leads to increased,Vascular endothelial dysfunction | August 19, 2023 20:14 |
| increased,Vascular endothelial dysfunction leads to Angiogenesis dysfunction | August 28, 2023 05:01 |
| Angiogenesis dysfunction leads to Increase, Vascular disrupting effects | August 28, 2023 05:03 |
Abstract
Data-driven analysis and pathway-based approaches contribute to reasonable arrangements of limited resources and laboratory tests of potential vascular disrupting compounds (pVDCs), which provides opportunities to save time and effort for toxicity research. With the widespread usage of chemicals related to vascular disrupting effects on a global scale, the concentrations generally reached up to micromolar range in environmental media and even in organisms. However, potential adverse effects and toxicity pathways of vascular disrupting compounds have not been systematically assessed. Therefore, it is necessary to review the current situation, formulate future research priorities, and characterize toxicity mechanisms. Results showed that this AOP may be invoked by effects on the inhibition of Nrf2. Downstream key events (KEs) include oxidative stress, Inflammation, Vascular endothelial dysfunction. KE relationships (KERs) lead to Angiogenesis dysfunction. The severity of adverse outcomes (vascular disrupting effects) would ultimately vary by anatomical region, organ system, and physiological state when an MIE is invoked. This study brought insights into facilitating the complement of AOP efficiently, as well as establishing toxicity pathways framework to inform risk assessment of emerging pVDCs.
AOP Development Strategy
Context
This AOP focuses on the vascular disrupting effect via inhibiting the Nrf2-signaling pathway. The abnormal expression of Nrf2 plays an important role in the vasculogenesis and angiogenesis. The postulated molecular initiating event (MIE) for this AOP may be invoked by effects on the inhibition of Nrf2. Downstream key events (KEs) include oxidative stress, Inflammation, Vascular endothelial dysfunction. KE relationships (KERs) leading to Angiogenesis dysfunction. The severity of adverse outcomes (vascular disrupting effects) would ultimately vary by anatomical region, organ system, and physiological state when an MIE is invoked. Furthermore, in order to elucidate the AOP of vascular disrupting effect better, the established AOPs are included.
Strategy
Summary of the AOP
Events:
Molecular Initiating Events (MIE)
Key Events (KE)
Adverse Outcomes (AO)
| Type | Event ID | Title | Short name |
|---|
| MIE | 1417 | NFE2/Nrf2 repression | NFE2/Nrf2 repression |
| KE | 1392 | Increase, Oxidative Stress | Increase, Oxidative Stress |
| KE | 2009 | Activation of inflammation pathway | Activation, inflammation pathway |
| KE | 1928 | increased,Vascular endothelial dysfunction | increased,Vascular endothelial dysfunction |
| KE | 2181 | Angiogenesis dysfunction | Angiogenesis dysfunction |
| AO | 2161 | Increase, Vascular disrupting effects | Increase, Vascular disrupting effects |
Relationships Between Two Key Events (Including MIEs and AOs)
| Title | Adjacency | Evidence | Quantitative Understanding |
|---|
| NFE2/Nrf2 repression leads to Increase, Oxidative Stress | adjacent | High | High |
| Increase, Oxidative Stress leads to Activation, inflammation pathway | adjacent | High | High |
| Activation, inflammation pathway leads to increased,Vascular endothelial dysfunction | adjacent | High | High |
| increased,Vascular endothelial dysfunction leads to Angiogenesis dysfunction | adjacent | High | High |
| Angiogenesis dysfunction leads to Increase, Vascular disrupting effects | adjacent | High | High |
| NFE2/Nrf2 repression leads to Activation, inflammation pathway | non-adjacent | High | High |
Network View
Prototypical Stressors
Life Stage Applicability
| Life stage | Evidence |
|---|---|
| All life stages | High |
Taxonomic Applicability
Sex Applicability
| Sex | Evidence |
|---|---|
| Mixed | High |
Overall Assessment of the AOP

The biological plausibility of KERs is strong due to the available mechanistic evidence present in studies from a wide variety of taxa. Nrf2 inhibition causes oxidative stress and a variety of cellular responses. Jeong et al. used a weight-of-evidence approach in analyzing TOXCAST data, and proposed the putative AOP pathway from MIE Increased Reactive Oxygen Species to KE Oxidative Stress to KE Increase, Inflammation. Support for the essentiality of the key events can be obtained from a wide diversity of taxonomic groups, with lab rats, mice, cell lines, and zebrafish. Some studies provided evidence including antagonism, knock-outs, or knock-ins to probe the necessity of MIE and KE. Furthermore, the AOP can be anticipated based on broader chemicals, which include PCBs, BPA, arsenic, cadmium, lead, and air pollution (PM2:5). The empirical support of KERs is largely found in toxicological studies derived from reference chemicals with dose-response and temporal concordance assessed. However, more studies are needed to explore the dose concordance, incidence concordance, and temporal concordance.
Domain of Applicability
- Life Stage Applicability
The AOPs are not life stage specific
- Taxonomic Applicability
|
Term |
Scientific Term |
Evidence |
Links |
|
Human |
Homo sapiens |
Low |
https://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?mode=Info&id=9606 |
|
Mouse |
Mus musculus |
High |
https://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?mode=Info&id=10090 |
|
Zebrafish |
Danio rerio |
High |
https://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?mode=Info&id=7955 |
- Sex Applicability
Mixed
Essentiality of the Key Events
The essentiality of KERs is strong due to various evidence from different controlled experimental designs with controls. Exposure to various chemical stressors has induced oxidative stress from Nrf2 inhibition. In this AOP we are focusing on the KERs between Nrf2 inhibition, oxidative stress, inflammation, vascular endothelial dysfunction, angiogenesis disfunction, vascular disrupting effects. Support for the essentiality of the key events can be obtained from a wide diversity of taxonomic groups, with lab rats, mice, cell lines, and zebrafish.
Evidence Assessment
The QWOE approach is an analytical method that utilizes causality criteria to assess the evidence-supported postulated AOP[4]. Firstly, the hypothesis of action was presented and the quantitative evaluation of evidence ranging from no evidence (0) to strong for each category (3, strong and −3, strong counter) utilizing the evolved MIEs, KEs, and KERs. Subsequently, a ranked importance-based numerical weight was assigned to Bradford Hill causal considerations, and the composite score and confidence score for MIEs, KEs, and entire AOP were evaluated.
| Assigned weight | Qualitative rating | |||||
| MIE | KE1 | KE2 | KE3 | KE4 | ||
| Biological plausibility | Some in vivo and in vitro evidence suggest that the chemicals can cause the vascular toxicity | |||||
| Essentiality empirical support | 0.4 | 1 | 1 | 1 | 1 | 1 |
| Dose and incidence concordance | 0.2 | 3 | 3 | 3 | 3 | 3 |
| Empirical support temporal concordance | 0.2 | 3 | 3 | 3 | 3 | 3 |
| Consistency across test systems | 0.1 | 3 | 3 | 3 | 3 | 3 |
| Analogy mutiple studies support KE and KER | 0.1 | 3 | 3 | 3 | 3 | 3 |
| Score | 1 | 2.2 | 2.2 | 2.2 | 2.2 | 2.2 |
| AOP Score | 0.733333 | |||||
Known Modulating Factors
| Modulating Factor (MF) | Influence or Outcome | KER(s) involved |
|---|---|---|
Quantitative Understanding
Optional field to provide quantitative weight of evidence descriptors.
Considerations for Potential Applications of the AOP (optional)
References
[1] ELLIS-HUTCHINGS R G, SETTIVARI R S, MCCOY A T, et al. Embryonic vascular disruption adverse outcomes: Linking high throughput signaling signatures with functional consequences [J]. Reproductive toxicology (Elmsford, NY), 2017, 70: 82-96.
[2] KLEINSTREUER N C, JUDSON R S, REIF D M, et al. Environmental impact on vascular development predicted by high-throughput screening [J]. Environ Health Perspect, 2011, 119(11): 1596-603.
[3] LIND L, ARAUJO J A, BARCHOWSKY A, et al. Key Characteristics of Cardiovascular Toxicants [J]. Environ Health Perspect, 2021, 129(9): 95001.
[4] BECKER R A, DELLARCO V, SEED J, et al. Quantitative weight of evidence to assess confidence in potential modes of action [J]. Regulatory toxicology and pharmacology : RTP, 2017, 86: 205-20.
[5] HE F, RU X, WEN T. NRF2, a Transcription Factor for Stress Response and Beyond [J]. International journal of molecular sciences, 2020, 21(13).
[6] KIM Y W, BYZOVA T V. Oxidative stress in angiogenesis and vascular disease [J]. Blood, 2014, 123(5): 625-31.
[7] KIM Y W, WEST X Z, BYZOVA T V. Inflammation and oxidative stress in angiogenesis and vascular disease [J]. Journal of molecular medicine (Berlin, Germany), 2013, 91(3): 323-8.
[8] DEANFIELD J E, HALCOX J P, RABELINK T J. Endothelial function and dysfunction: testing and clinical relevance [J]. Circulation, 2007, 115(10): 1285-95.
[9] GODO S, SHIMOKAWA H. Endothelial Functions [J]. Arteriosclerosis, thrombosis, and vascular biology, 2017, 37(9): e108-e14.
[10] INCALZA M A, D'ORIA R, NATALICCHIO A, et al. Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases [J]. Vascul Pharmacol, 2018, 100: 1-19.
[11] WEI Y, GONG J, XU Z, et al. Nrf2 promotes reparative angiogenesis through regulation of NADPH oxidase-2 in oxygen-induced retinopathy [J]. Free radical biology & medicine, 2016, 99: 234-43.
[12] ZHONG X, QIU J, KANG J, et al. Exposure to tris(1,3-dichloro-2-propyl) phosphate (TDCPP) induces vascular toxicity through Nrf2-VEGF pathway in zebrafish and human umbilical vein endothelial cells [J]. Environmental pollution (Barking, Essex : 1987), 2019, 247: 293-301.
[13] WEI Y, GONG J, XU Z, et al. Nrf2 in ischemic neurons promotes retinal vascular regeneration through regulation of semaphorin 6A [J]. Proceedings of the National Academy of Sciences of the United States of America, 2015, 112(50): E6927-36.
[14] WEI Y, GONG J, THIMMULAPPA R K, et al. Nrf2 acts cell-autonomously in endothelium to regulate tip cell formation and vascular branching [J]. Proceedings of the National Academy of Sciences of the United States of America, 2013, 110(41): E3910-8.
[15] XU Z, WEI Y, GONG J, et al. NRF2 plays a protective role in diabetic retinopathy in mice [J]. Diabetologia, 2014, 57(1): 204-13.
[16] WEI Y, GONG J, YOSHIDA T, et al. Nrf2 has a protective role against neuronal and capillary degeneration in retinal ischemia-reperfusion injury [J]. Free radical biology & medicine, 2011, 51(1): 216-24.
[17] CIMINO F, SPECIALE A, ANWAR S, et al. Anthocyanins protect human endothelial cells from mild hyperoxia damage through modulation of Nrf2 pathway [J]. Genes & nutrition, 2013, 8(4): 391-9.
[18] CHEN B, LU Y, CHEN Y, et al. The role of Nrf2 in oxidative stress-induced endothelial injuries [J]. The Journal of endocrinology, 2015, 225(3): R83-99.
[19] ISHIKADO A, SONO Y, MATSUMOTO M, et al. Willow bark extract increases antioxidant enzymes and reduces oxidative stress through activation of Nrf2 in vascular endothelial cells and Caenorhabditis elegans [J]. Free radical biology & medicine, 2013, 65: 1506-15.
[20] HAN S G, HAN S S, TOBOREK M, et al. EGCG protects endothelial cells against PCB 126-induced inflammation through inhibition of AhR and induction of Nrf2-regulated genes [J]. Toxicol Appl Pharmacol, 2012, 261(2): 181-8.
[21] SAHA S, BUTTARI B, PANIERI E, et al. An Overview of Nrf2 Signaling Pathway and Its Role in Inflammation [J]. Molecules (Basel, Switzerland), 2020, 25(22).
[22] ZHONG X, YU Y, WANG C, et al. Hippocampal proteomic analysis reveals the disturbance of synaptogenesis and neurotransmission induced by developmental exposure to organophosphate flame retardant triphenyl phosphate [J]. J Hazard Mater, 2021, 404(Pt B): 124111.
[23] ZHONG X, WU J, KE W, et al. Neonatal exposure to organophosphorus flame retardant TDCPP elicits neurotoxicity in mouse hippocampus via microglia-mediated inflammation in vivo and in vitro [J]. Archives of toxicology, 2020, 94(2): 541-52.
[24] TARANTINI S, VALCARCEL-ARES M N, YABLUCHANSKIY A, et al. Nrf2 Deficiency Exacerbates Obesity-Induced Oxidative Stress, Neurovascular Dysfunction, Blood-Brain Barrier Disruption, Neuroinflammation, Amyloidogenic Gene Expression, and Cognitive Decline in Mice, Mimicking the Aging Phenotype [J]. The journals of gerontology Series A, Biological sciences and medical sciences, 2018, 73(7): 853-63.
[25] ZHENG S, WANG Y, GUO W, et al. FOXO6 transcription inhibition of CTRP3 promotes OGD/R-triggered cardiac microvascular endothelial barrier disruption via SIRT1/Nrf2 signaling [J]. Folia morphologica, 2023.
[26] WANG D P, KANG K, SUN J, et al. URB597 and Andrographolide Improve Brain Microvascular Endothelial Cell Permeability and Apoptosis by Reducing Oxidative Stress and Inflammation Associated with Activation of Nrf2 Signaling in Oxygen-Glucose Deprivation [J]. Oxidative medicine and cellular longevity, 2022, 2022: 4139330.
[27] ZHANG Q, LIU J, DUAN H, et al. Activation of Nrf2/HO-1 signaling: An important molecular mechanism of herbal medicine in the treatment of atherosclerosis via the protection of vascular endothelial cells from oxidative stress [J]. Journal of advanced research, 2021, 34: 43-63.
[28] ZHANG H, YUAN B, HUANG H, et al. Gastrodin induced HO-1 and Nrf2 up-regulation to alleviate H2O2-induced oxidative stress in mouse liver sinusoidal endothelial cells through p38 MAPK phosphorylation [J]. Brazilian journal of medical and biological research = Revista brasileira de pesquisas medicas e biologicas, 2018, 51(10): e7439.
[29] ZHANG C, KONG X, MA D. miR-141-3p inhibits vascular smooth muscle cell proliferation and migration via regulating Keap1/Nrf2/HO-1 pathway [J]. IUBMB life, 2020, 72(10): 2167-79.
[30] YANG K, SONG H, YIN D. PDSS2 Inhibits the Ferroptosis of Vascular Endothelial Cells in Atherosclerosis by Activating Nrf2 [J]. Journal of cardiovascular pharmacology, 2021, 77(6): 767-76.
[31] SHA W, ZHAO B, WEI H, et al. Astragalus polysaccharide ameliorates vascular endothelial dysfunction by stimulating macrophage M2 polarization via potentiating Nrf2/HO-1 signaling pathway [J]. Phytomedicine : international journal of phytotherapy and phytopharmacology, 2023, 112: 154667.
[32] LIU L, WANG R, XU R, et al. Procyanidin B2 ameliorates endothelial dysfunction and impaired angiogenesis via the Nrf2/PPARγ/sFlt-1 axis in preeclampsia [J]. Pharmacological research, 2022, 177: 106127.
[33] LI H, ZHUANG W, XIONG T, et al. Nrf2 deficiency attenuates atherosclerosis by reducing LOX-1-mediated proliferation and migration of vascular smooth muscle cells [J]. Atherosclerosis, 2022, 347: 1-16.
[34] ASHINO T, YAMAMOTO M, YOSHIDA T, et al. Redox-sensitive transcription factor Nrf2 regulates vascular smooth muscle cell migration and neointimal hyperplasia [J]. Arteriosclerosis, thrombosis, and vascular biology, 2013, 33(4): 760-8.
[35] WANG Q, LIU Y, GUO J, et al. Microcystin-LR induces angiodysplasia and vascular dysfunction through promoting cell apoptosis by the mitochondrial signaling pathway [J]. Chemosphere, 2019, 218: 438-48.
[36] UNGVARI Z, TARANTINI S, KISS T, et al. Endothelial dysfunction and angiogenesis impairment in the ageing vasculature [J]. Nature reviews Cardiology, 2018, 15(9): 555-65.
[37] ALEXANDER Y, OSTO E, SCHMIDT-TRUCKSäSS A, et al. Endothelial function in cardiovascular medicine: a consensus paper of the European Society of Cardiology Working Groups on Atherosclerosis and Vascular Biology, Aorta and Peripheral Vascular Diseases, Coronary Pathophysiology and Microcirculation, and Thrombosis [J]. Cardiovascular research, 2021, 117(1): 29-42.