
This AOP is licensed under the BY-SA license. This license allows reusers to distribute, remix, adapt, and build upon the material in any medium or format, so long as attribution is given to the creator. The license allows for commercial use. If you remix, adapt, or build upon the material, you must license the modified material under identical terms.
AOP: 526
Title
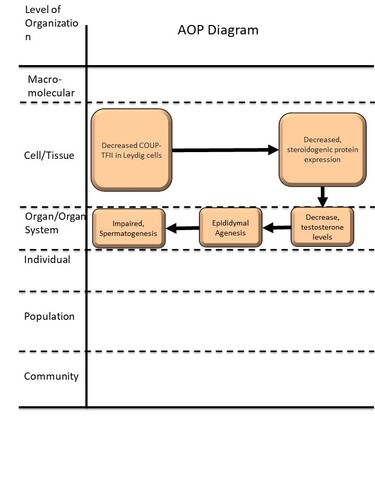
Decreased, Chicken Ovalbumin Upstream Promoter Transcription Factor II (COUP-TFII) leads to Impaired, Spermatogenesis
Short name
Graphical Representation
Point of Contact
Contributors
- John Frisch
Coaches
OECD Information Table
| OECD Project # | OECD Status | Reviewer's Reports | Journal-format Article | OECD iLibrary Published Version |
|---|---|---|---|---|
This AOP was last modified on October 30, 2024 15:15
Revision dates for related pages
| Page | Revision Date/Time |
|---|---|
| Decreased, Chicken Ovalbumin Upstream Promoter Transcription Factor II (COUP-TFII) | December 03, 2024 10:26 |
| Decreased, steroidogenic protein expression | December 03, 2024 10:27 |
| Decrease, circulating testosterone levels | January 27, 2025 03:37 |
| Epididymal agenesis | December 03, 2024 10:28 |
| Impaired, Spermatogenesis | April 10, 2024 17:41 |
| Decreased COUP-TFII in Leydig cells leads to Decreased, steroidogenic protein expression | December 03, 2024 10:29 |
| Decreased, steroidogenic protein expression leads to Decrease, circulating testosterone levels | December 03, 2024 10:29 |
| Decrease, circulating testosterone levels leads to Epididymal agenesis | December 03, 2024 10:29 |
| Epididymal agenesis leads to Impaired, Spermatogenesis | December 03, 2024 10:31 |
Abstract
Impaired spermatogenesis is an adverse outcome often observed among a group of male reproductive abnormalities caused by organ malformation (epididymis, vas deferens, seminal vesicles, prostate, external genitalia) during development (Drake et al. 2009; Palermo et al. 2021). These reproductive abnormalities have been observed in studies of laboratory mice and rats exposed to phthalates during in utero development, in attempts to understand the gene expression/inhibition, hormone levels, and other factors leading to the observed adverse outcomes. Studies in laboratory mammals have allowed researchers to target the role of individual genes by knockout gene studies, and target critical developmental windows by timed exposure to toxicants, to explore the mechanisms leading to reproductive defects similar to human birth defects observed in clinical studies (Review in Foster 2006). Although a molecular initiating event isn’t well established, decreased Chicken Ovalbumin Upstream Promoter Transcription Factor II (COUP-TFII)) gene expression has been linked to decreased expression of genes coding for enzymes involved in steroidogenesis and decreased testosterone levels in mammals (Qin et al. 2008; van den Driesche et al. 2012; Mendoza-Villarroel et al. 2014). One adverse outcome of decreased testosterone, and the focus of this adverse outcome pathway, is epididymal agenesis, and resulting impaired spermatogenesis (Mahood et al. 2007; Qin et al. 2008; Kim et al. 2010). Impaired spermatogenesis results in decreased sperm counts, as well as decreases in the number of sperm capable of fertilization (Barlow and Foster 2003).
AOP Development Strategy
Context
This Adverse Outcome Pathway (AOP) was developed as part of an Environmental Protection Agency effort to represent putative AOPs from peer-reviewed literature which were heretofore unrepresented in the AOP-Wiki. The originating work for this AOP was: Palermo, C.M., Foreman, J.E., Wikoff, D.S., and Lea, I. 2021. Development of a putative adverse outcome pathway network for male rat reproductive tract abnormalities with specific considerations for the androgen sensitive window of development. Current Research in Toxicology 2: 254–271. This publication, and the work cited within, were used create and support this AOP and its respective KE and KER pages.
Phthalates are of increasing human health concern because of increased use and accumulating evidence of disruption of reproductive development in vertebrates. First detected in laboratory mammals, exposure to phthalates and other toxicants in utero when male sexual differentiation is occurring have resulted in increased malformation of reproductive organs, failure of male characteristics to develop, and failure of proper positioning of organs (ex. hypospadias and cryptorchidism). Clinical studies in humans have used laboratory mammal data to help understand and treat conditions exhibited by individual people. This AOP focuses on the pathway leading to impaired to spermatogenesis, via abnormal formation of the epididymis, decreased testosterone levels, and initiated by decreased Chicken Ovalbumin Upstream Promoter Transcription Factor II (COUP-TFII) gene expression and subsequent disrupted signaling for steroidogenesis.
The focus of the originating work was to use an AOP framework to integrate lines of evidence from multiple disciplines based on evolving guidance developed by the Organization for Economic Cooperation and Development (OECD). Palermo et al. (2021) provided network analysis based on two literature searches: 1. rodent male reproductive development abnormalities using key terms; 2. effects of low molecular weight phthalates (LMWPs) during the rodent male programming window (MPW) of development. Relevant key events and key event relationships were narrowed by focusing on empirical studies related to ‘rat phthalate syndrome’ which resulted in 3 recommended Adverse Outcome Pathways: 1. INSL expression to cryptorchidism (see AOP 528 for related content); 2. COUP-TFII expression to hypospadias (see AOP 527 for related content); 3. COUP-TFII expression to altered sperm maturation (see this AOP 526 for related content).
Strategy
The originating authors conducted a literature search to develop a database of publications categorized by discipline or field of study: toxicology, epidemiology, exposure, and gene-environment interaction. The literature search relied on standard search engines such as Web of Science and Google Scholar, and the search strategy focused on toxicants known to lead to male reproductive abnormalities in organisms. The originating authors reviewed references from individual citations to identify additional studies not captured through the literature search itself. They then included all relevant publications through 2023.
The scope of the aforementioned EPA project was limited to re-representing the AOP(s) as presented in the originating publication. The literature used to support this AOP and its constituent pages began with the originating publication and followed to the primary, secondary, and tertiary works cited therein. KE and KER page creation and re-use was determined using Handbook principles where page re-use was preferred.

The authors of AOP 526 also referred to existing AOP-wiki content on disruption of steroidogenesis pathways, especially work by Gary Klinefelter (ex. AOP 70, 71). We found existing Adverse Outcome Pathway content documented different series of key events then the pathways provided by Palermo et al. (2021), and therefore initiated AOP 526 and updated existing AOP-wiki key events when available.
Summary of the AOP
Events:
Molecular Initiating Events (MIE)
Key Events (KE)
Adverse Outcomes (AO)
| Type | Event ID | Title | Short name |
|---|
| KE | 656 | Decreased, Chicken Ovalbumin Upstream Promoter Transcription Factor II (COUP-TFII) | Decreased COUP-TFII in Leydig cells |
| KE | 647 | Decreased, steroidogenic protein expression | Decreased, steroidogenic protein expression |
| KE | 1690 | Decrease, circulating testosterone levels | Decrease, circulating testosterone levels |
| KE | 2212 | Epididymal agenesis | Epididymal agenesis |
| AO | 1758 | Impaired, Spermatogenesis | Impaired, Spermatogenesis |
Relationships Between Two Key Events (Including MIEs and AOs)
| Title | Adjacency | Evidence | Quantitative Understanding |
|---|
| Decreased COUP-TFII in Leydig cells leads to Decreased, steroidogenic protein expression | adjacent | High | Not Specified |
| Decreased, steroidogenic protein expression leads to Decrease, circulating testosterone levels | adjacent | High | Not Specified |
| Decrease, circulating testosterone levels leads to Epididymal agenesis | adjacent | High | Not Specified |
| Epididymal agenesis leads to Impaired, Spermatogenesis | adjacent | High | Not Specified |
Network View
Prototypical Stressors
Life Stage Applicability
| Life stage | Evidence |
|---|---|
| During development and at adulthood | High |
Taxonomic Applicability
| Term | Scientific Term | Evidence | Link |
|---|---|---|---|
| mammals | mammals | Moderate | NCBI |
Sex Applicability
| Sex | Evidence |
|---|---|
| Male | High |
Overall Assessment of the AOP
|
1. Support for Biological Plausibility of Key Event Relationships: Is there a mechanistic relationship between KEup and KEdown consistent with established biological knowledge? |
|
|
Key Event Relationship (KER) |
Level of Support Strong = Extensive understanding of the KER based on extensive previous documentation and broad acceptance. |
|
Relationship 3167: Decreased COUP-TFII in Leydig cells leads to Decreased, steroidogenic protein expression |
Strong support. The relationship between decrease in COUP-TFII expression and decreased steroidogenic enzymes (ex. CYP11, CYP17, P450scc, SR-B1, StAR) is broadly accepted and consistently supported across lab mice, lab rats, and clinical human studies. |
|
Relationship 3168: Decreased, steroidogenic protein expression leads to Decrease, testosterone levels |
Strong support. The relationship between decreased steroidogenic enzymes and decreased testosterone is broadly accepted and consistently supported across lab mice, lab rats, and clinical human studies. |
|
Relationship 3169: Decrease, testosterone levels leads to Epididymal Agenesis |
Strong support. Decreased testosterone levels have consistently been linked to epididymal agenesis and consistently supported across lab mice, lab rats, and clinical human studies. |
|
Relationship 3170: Epididymal Agenesis leads to Impaired, Spermatogenesis |
Strong support. Epididymal agenesis and improper formation of the epididymis has been shown to results in impaired spermatogenesis (decreased sperm counts and function) across lab mice, lab rats, and clinical human studies. |
|
Overall |
Strong support. Extensive understanding of the relationships between events from empirical studies from a variety of taxa, including frequent testing in lab mammals. |
Domain of Applicability
Life Stage: Problems first can be observed during development, with adverse outcome manifesting in mature individuals.
Sex: Applies to males.
Taxonomic: Appears to be present broadly in mammals, with most representative studies in mammals (humans, lab mice, lab rats).
Essentiality of the Key Events
|
2. Essentiality of Key Events: Are downstream KEs and/or the AO prevented if an upstream KE is blocked? |
|
|
Key Event (KE) |
Level of Support Strong = Direct evidence from specifically designed experimental studies illustrating essentiality and direct relationship between key events. Moderate = Indirect evidence from experimental studies inferring essentiality of relationship between key events due to difficulty in directly measuring at least one of key events. |
|
KE 656: Decreased COUP-TFII in Leydig cells |
Moderate support. Decrease in COUP-TFII expression has been linked to decreased steroidogenic enzymes (ex. CYP11, CYP17, P450scc, SR-B1, StAR). Evidence is available from toxicant, gene-knockout, and protein studies. Best evidence for essentiality of COUP-TFII expression is in gene-knockout studies in which wild-type individuals retain normal steroidogenic enzyme levels, while individuals with diminished COUP-TFII expression have decreased steroidogenic enzyme levels. |
|
KE 647 Decreased, steroidogenic protein expression |
Strong support. Decreased expression of steroidogenic enzymes (ex. CYP11, CYP17, P450scc, SR-B1, StAR is linked to decreased testosterone levels. Evidence is available from toxicant, gene-knockout, and protein studies. Best evidence for essentiality of steroidogenic enzymes is in toxicant studies caused decreased steroidogenic protein expression and testosterone levels, and after cessation of toxicant exposure steroid protein expression and testosterone levels return to normal. |
|
KE 1690 Decrease, testosterone levels |
Moderate support. Decreases in testosterone is linked with epididymal agenesis and abnormal development of epididymides. Evidence is available from toxicant and histology studies. Best evidence for the essentiality of testosterone levels is when supplemental testosterone treatment restores normal epididymis formation. |
|
KE 2212 Epididymal Agenesis |
Strong support. Malformed epididymides and epididymal agenesis is linked to impaired spermatogenesis. Evidence is available from toxicant and histology studies. As the epididymis is a primary location for sperm development, abnormal sperm lacking proper function, sperm counts, and assessment of reproductive function show the essentiality of proper epididymis formation. |
|
AO 1758 Impaired, Spermatogenesis |
This is the final event of the AOP. |
|
Overall |
Moderate to strong support. Direct evidence from empirical studies from laboratory mammals for most key events, with more inferential evidence for gene expression and protein studies. |
Evidence Assessment
|
3. Empirical Support for Key Event Relationship: Does empirical evidence support that a change in KEup leads to an appropriate change in KEdown? |
|
|
Key Event Relationship (KER) |
Level of Support Strong = Experimental evidence from exposure to toxicant shows consistent change in both events across taxa and study conditions. |
|
Relationship 3167: Decreased COUP-TFII in Leydig cells leads to Decreased, steroidogenic protein expression |
Strong support. Decreases in COUP-TFII expression lead to decreased steroidogenic enzymes (ex. CYP11, CYP17, P450scc, SR-B1, StAR, primarily from studies examining COUP-TFII knock-out genes, as well as changes in gene expression/protein levels after exposure to chemical stressors. |
|
Relationship 3168: Decreased, steroidogenic protein expression leads to Decrease, testosterone levels |
Strong support. Decreases in steroidogenesis enzymes lead to decreases in testosterone levels, primarily from studies measuring gene expression and correlation to protein and hormone levels. |
|
Relationship 3169: Decrease, testosterone levels leads to Epididymal Agenesis |
Strong support. Decreases in testosterone have resulted in malformation and agenesis of epididymides through measurement of hormone levels, and resulting issues in reproductive tissue formation. |
|
Relationship 3170: Epididymal Agenesis leads to Impaired, Spermatogenesis |
Strong support. Malformation of epididymides directly impacts ability of the organ to develop normal numbers of functional sperm. |
|
Overall |
Strong support. Evidence from empirical studies shows consistent change in both events from a variety of taxa, including frequent testing in lab mammals. |
Known Modulating Factors
| Modulating Factor (MF) | Influence or Outcome | KER(s) involved |
|---|---|---|
Quantitative Understanding
Considerations for Potential Applications of the AOP (optional)
References
Barlow, N.J. and Foster, P.M.D. 2003. Pathogenesis of Male Reproductive Tract Lesions from Gestation Through Adulthood Following in Utero Exposure to Di(n-butyl) Phthalate. Toxicologic Pathology 31:397–410.
Drake, A.J., van den Driesche, S., Scott, H.M., Hutchinson, G.R., Seckl, J.R. and Sharpe, R.M. 2009. Glucocorticoids Amplify Dibutyl Phthalate-Induced Disruption of Testosterone Production and Male Reproductive Development. Endocrinology 150(11): 5055–5064.
Foster, P.M.D. 2006. Disruption of reproductive development in male rat offspring following in utero exposure to phthalate esters. International Journal of Andrology 29: 140–147.
Kim, T.S., Jung, K.K., Kim, S.S., Kang, I.H., Baek, J.H., Nam, H.-S., Hong, S.-K., Lee, B.M., Hong, J.T., Oh, K.W., Kim, H.S., Han, S.Y., and Kang, T.S. 2010. Effects of in Utero Exposure to DI(n-Butyl) Phthalate on Development of Male Reproductive Tracts in Sprague-Dawley Rats. Journal of Toxicology and Environmental Health, Part A 73(21-22): 1544-1559.
Mahood, I.K., Scott, H.M., Brown, R., Hallmark, N., Walker, M., and Sharpe, R.M. 2007. In Utero Exposure to Di(n-butyl) Phthalate and Testicular Dysgenesis: Comparison of Fetal and Adult End Points and Their Dose Sensitivity. Environmental Health Perspectives 115 (supplement 1): 55-61.
Mendoza-Villarroel, R.E., Robert, N.M., Martin, L.J., Brousseau, C., and Tremblay, J.J. 2014. The Nuclear Receptor NR2F2 Activates Star Expression and Steroidogenesis in Mouse MA-10 and MLTC-1 Leydig Cells. Biology of Reproduction 91(1) Article 26: 1-12.
Palermo, C.M., Foreman, J.E., Wikoff, D.S., and Lea, I. 2021. Development of a putative adverse outcome pathway network for male rat reproductive tract abnormalities with specific considerations for the androgen sensitive window of development. Current Research in Toxicology 2: 254–271.
Qin, J., Tsai, M.-J., and Tsai S.Y. 2008. Essential Roles of COUP-TFII in Leydig Cell Differentiation and Male Fertility. Public Library of Science One 3(9): e3285.
van den Driesche, S., Walker, M., McKinnel, C., Scott, HM., Eddie, S.L., Mitchell, R.T., Seckl, J.R., Drake, A.J., Smith, L.B., Anderson, R.A., and Sharpe, R.M. 2012. Proposed Role for COUP-TFII in Regulating Fetal Leydig Cell Steroidogenesis, Perturbation of Which Leads to Masculinization Disorders in Rodents. Public Library of Science One 7(5): e37064.