
This AOP is licensed under the BY-SA license. This license allows reusers to distribute, remix, adapt, and build upon the material in any medium or format, so long as attribution is given to the creator. The license allows for commercial use. If you remix, adapt, or build upon the material, you must license the modified material under identical terms.
AOP: 202
Title
Inhibitor binding to topoisomerase II leading to infant leukaemia
Short name
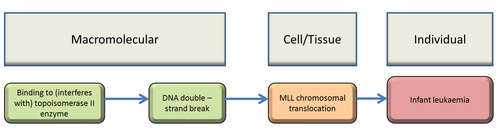
Graphical Representation
Point of Contact
Contributors
- Andrea Terron
- Clemens Wittwehr
Coaches
OECD Information Table
| OECD Project # | OECD Status | Reviewer's Reports | Journal-format Article | OECD iLibrary Published Version |
|---|---|---|---|---|
| 1.53 | WPHA/WNT Endorsed | Scientific Review | iLibrary link |
This AOP was last modified on April 29, 2023 16:02
Revision dates for related pages
| Page | Revision Date/Time |
|---|---|
| Binding to (interferes with) topoisomerase II enzyme | August 01, 2022 08:24 |
| MLL chromosomal translocation | August 01, 2022 08:27 |
| Infant leukaemia | August 01, 2022 08:33 |
| Increase, DNA double-strand breaks | November 13, 2023 10:30 |
| Binding, topoisomerase II leads to Increase, DSB | August 01, 2022 08:34 |
| Increase, DSB leads to MLL translocation | August 01, 2022 08:35 |
| MLL translocation leads to IFL | August 01, 2022 08:39 |
| Etoposide | July 27, 2022 03:55 |
| Bioflavonoids | July 27, 2022 04:01 |
| Chlorpyrifos | July 27, 2022 04:02 |
| etoposide quinone | July 27, 2022 04:04 |
Abstract
Infant leukaemia is a rare haematological disease (1 in 106 newborns, accounting for 10% of all childhood acute lymphoblastic leukaemias (ALL)) manifesting soon after birth (<1 year) and having a poor prognosis (Sanjuan-Pla et al 2015). Compared to the more frequent childhood leukaemia, infant leukaemia show distinct features:
- An early neonatal onset linked to its plausible origin as a ‘intrauterine developmental disease’ (Greaves 2015; Sanjuan-Pla et al 2015);
- Rearrangements of the mixed-lineage leukaemia (MLL; KMT2A) gene on the q23 band of chromosome 11, as the hallmark genetic abnormality (Joannides and Grimwade 2010);
- However, MLL is not the only translocation gene; for infant ALL, about 60-80% carry an MLL rearrangement (Sam et al.2012; Jansen et al.2007) and the percentage for infant (including youngs and middle age adults) acute myeloid leukaemia (AML) is about 40 %;
- The MLL rearrangement at an early stage of development; the likely target cells (still unidentified) are the hematopoietic stem and progenitor cells (HSPC) in fetal liver and/or earlier (mesenchymal) stem cells in embryonic mesoderm (Bueno et al 2009; Menendez et al 2009);
- The infant MLL-rearranged leukaemia carries less somatic mutations (1.3 vs 6.5/case) than the childhood disease (Andersson et al 2015; Dobbins et al 2013), pointing to the lack of a “second hit” and suggesting a “one big hit” origin.
Following these distinct features a Molecular Initiating Event (MIE), two Key Event (KE) and an Adverse Outcome (AO) were identified. The MIE was identified as " DNA topoisomerase II poisons (interfers with) topo II enzyme" and epidemiological studies suggest that exposure to topoisomerases II poisons may be involved in generation of the two KEs, DNA double strand break and MLL chromosomal rearrangement.
Overall, based on the available evidence, infant leukaemia pathogenesis originates from a single, severe hit to a target cell during early intrauterine development. Whereas the limited epidemiological studies do not allow any firm conclusion on a possible role for chemicals in infant leukaemia (Pombo-de-Oliveira et al 2006; Ferreira et al 2013), exposures to chemicals able to induce MLL rearrangements through topoisomerase II (TopoII) “poison”, particularly etoposide and other TopoII “poisons”, including some bioflavonoids, have been suggested as agents promoting the driving genetic oncogenic event. Experimental models for infant leukaemia have been developed, but a wholly satisfactory model reproducing the phenotype and latency is not yet available.
Nevertheless, the anticancer drug etoposide can be considered as a model chemical for DNA topoisomerase “poison”. Acute leukaemia is an adverse effect recorded in etoposide-treated patients, showing MLL rearrangements that are in many ways analogous to those in infant leukaemia (Bueno et al 2009; Joannides et al 2010, 2011). Therefore, the proposed AOP is supported by a number of convincing inferential evidences by means of using etoposide as a model compound to empirically support the linkage between the proposed molecular initiating event (MIE) and the adverse outcome (AO). In the meanwhile, this AOP identifies several knowledge gaps, the main ones being the identification of the initiating cell and the investigation of TopoII poisons in a robust model; thus, the present AOP may be modified in future on the basis of new evidence. The authors recognize that additional elements are limiting the strenght of this AOP. Although a strong empirical support exists for the direct link between the MIE and the DNA double strand break and between this KE and the MLL translocation, the empirical support for the indirect link between MLL translocation and the AO is mainly based on one chemical stressor and that essentiality data are also limited and difficult to generate. The biological plausibility for the KERs is considered high for the initial step but is only moderate for the final step because of the uncertainties associated with lack of knowledge in the final step of the disease and lack of appropriate models able to fully recapitulate the disease. The empirical support for the KERs is overall considered moderate, as the relevant data only exist for etoposide and evidence is mainly indirect and based on the evidence from the therapy associated acute myeloid leukaemia. Therefore, the overall biological plausibility is considered moderate and the empirical support is also moderate
AOP Development Strategy
Context
Infant leukaemia (<1 year old) is a rare disease of developmental origin distinct from adult and childhood leukaemias which fit the classical two-hit cancer model. Both genetic and haematological studies indicate an in utero origin at an early phase of foetal development. Investigation of identical twin pairs with infant leukaemia provided evidence of in-utero transfer of leukemic cells from one twin to the other (Ford AM, 1993), and the in-utero origin of this cancer was confirmed by retrospective analyses of neonatal blood spots from affected infants (Gale KB, 1997). The high concordance rate for leukaemia in monozygotic twins and the short latency of the disease suggest that MLL rearrangement in fetal hematopoietic stem cells causes infant leukaemia (Nanya M, 2015). Rearrangements of the mixed lineage leukemia (MLL) gene producing abnormal fusion protein are the most frequent genetic/molecular hallmarks in infant B-cell ALL. In small epidemiological studies, mother/foetus pesticide exposure has been associated with infant leukaemia; however, strength of evidence and power of these studies are weak at best. Despite recent advances in the pathogenesis of pediatric leukemia, surrogate models such as in vitro, ex vivo or animals in vivo do not reproduce the human disease sufficiently and they suffer from difficulties in interpretation and extrapolation of findings and from the intrinsic limitation in cancer bio-assay design to cover relevant window of exposure. This adverse outcome pathway (AOP) is based substantially on an analogous disease – secondary acute leukaemia caused by etoposide, a topoisomerase II (TopoII) poison –, and on cellular and animal models. The topo II inhibitor, Etoposide, induces DNA double-strand breaks between the S and the G2/M phases of the cell cycle and is related to the post treatment occurence of the acutemyeloid leukaemia, which is showing a similar pattern of genetic changes as observed in the Infant Leukaemia (IFL) disease. Indeeed, the hallmark of the IFL and acute myeloid leukaemia is the formation of MLL gene rearrangements (MLLr) via TopoII poisoning, leading to fusion genes and eventually acute leukaemia by global (epi)genetic dysregulation. Current knowledge supports the possibility that MLL-rearrangment in infant leukaemia is caused by transplacental exposure to topo2 poisons. Although it is considered unusual for a pregnant woman to be directly exposed to drugs such as etoposide, other compounds presents in the environment may exert similar effects, and this is considered toxicologically relevant for risk assessment (Nanya M, 2015). This AOP condenses molecular, pathological, regulatory, clinical and epidemiological knowledge in a pragmatic framework with the aspiration of focussing on human specific hazard in the risk assessment process. This AOP enables to identify important gaps of knowledge relevant to risk assessment, including the specific embryonic target cell during the short and spatially restricted period of susceptibility and the role of (epi)genetic features modifying initiation and progression of the disease. Furthermore, this AOP informs on a potential integrated approach to testing and assessment (IATA) to address the risk caused by environmental chemicals in the future and represents a transparent and weight of evidence based tool to define the plausible causative mechanism necessary for the interpretation and integration of epidemiological studies in the process of risk assessment. This AOP was first developed by the EFSA PPR Panel as part of a Scientific Opinion and published in the EFSA Journal 2017;15(3):4691DOI: 10.2903/j.efsa.2017.4691. A copyright for figures and for most of the references included in this AOP was delt with in the EFSA Scientific Opinion. In addition, EFSA granted a research project for assessing in vitro and in vivo the potential genotoxic contribution of etoposide, Permethrin and Chlorpyrifos in human hematopoietic stem and progenitor cells (HSPCs) at different ontogeny stages, spanning from embryonic to adult HSPCs, with a special emphasis in their ability to induce MLL breaks/damage (Rodriguez et al. 2020)
Strategy
Summary of the AOP
Events:
Molecular Initiating Events (MIE)
Key Events (KE)
Adverse Outcomes (AO)
| Type | Event ID | Title | Short name |
|---|
| MIE | 1252 | Binding to (interferes with) topoisomerase II enzyme | Binding, topoisomerase II |
| KE | 1461 | Increase, DNA double-strand breaks | Increase, DSB |
| KE | 1253 | MLL chromosomal translocation | MLL translocation |
| AO | 1254 | Infant leukaemia | IFL |
Relationships Between Two Key Events (Including MIEs and AOs)
| Title | Adjacency | Evidence | Quantitative Understanding |
|---|
| Binding, topoisomerase II leads to Increase, DSB | adjacent | High | Not Specified |
| Increase, DSB leads to MLL translocation | adjacent | High | Not Specified |
| MLL translocation leads to IFL | adjacent | High | Not Specified |
Network View
Prototypical Stressors
Life Stage Applicability
| Life stage | Evidence |
|---|---|
| Embryo | High |
Taxonomic Applicability
| Term | Scientific Term | Evidence | Link |
|---|---|---|---|
| human | Homo sapiens | High | NCBI |
Sex Applicability
| Sex | Evidence |
|---|---|
| Unspecific | High |
Overall Assessment of the AOP
Direct studies in humans are difficult or impossible to conduct and one has to resort to surrogate in vitro or ex vivo studies or to animal models, which necessarily are associated with difficulties in interpretation and extrapolation. Thus, what is described in this overall assessment is based largely on inferences from analogous diseases using tool chemicals able to reproduce the biological basis of the disease (especially etoposide, a Topoisomerases II poison-caused acute leukaemia in children or adults) or from cellular and animal models. All cells have the two major forms of topoisomerases. Topoisomerases are able to alter the topological state of the DNA and toposisomerases are important targets for may chemoterapeutic agent and antibiotics (e.g. Fluoroquinolones). For examples, drugs that inhibit DNA topoisomerase II, such as doxorubicin and etoposide, can cause DNA damage.These agents prevent the DNA-resealing step which is normally catalyzed by topoisomerases. This AOP is mainly using etoposide for the KER empirical support as only for this agent an indirect link between MLL translocation and secondary leukaemia in cancer patients exist and the sequence of KEs (including the MIE) can be used to support an AOP specific for the AO i.e. Infant Leukaemia. Consequently, the biological plausibility for the KERs is considered high only for the initial step but is only moderate for the final step because of the uncertainties associated with lack of knowledge in the final step of the disease and lack of appropiate models able to fully recapitualte the disease. The empirical support for the KERs is overall considered moderate, as the relevant data only exist for etoposide and evidence are mainly indirect and based on the evidence from the therapy associated acute myeloid leukaemia.
1. Concordance of dose-response relationship
The only study in mice (Nanya et al 2016; conducted following in-utero exposure)) has shown that the dose of 0.5 mg/kg etoposide (day 13.5 of pregnancy) does not result in measurable etoposide concentration in foetal liver hematopoietic stem cells (HSCs) whereas the dose of 10 mg/kg leads to a maximal concentration of 5 µM. A statistically significant increase in double strand break (DSBs) and MLL translocation was observed at a dose of 1 mg/kg, which would result in a concentration of 0.5 µM by linear extrapolation. In treatment-related acute human myeloid leukaemia, various treatment schedules in adults and children give rise to etoposide concentrations between (roughly) <1 µM (through to >150 µM (peak). There are no adequate experimental systems to study dose-response and response-response relationships across MIE, KEs and AO in a single model.
2. Temporal concordance among the MIE, KEs and AO
There are no serious doubts about temporal concordance among MIE, KEs and AO. It is very difficult to see any other sequence of events (among this AOP), which would bring the AO into effect. Another matter is that it has never been shown in human pregnancy (or will be reliably or robustly demonstrated in the foreseeable future). In this respect, it is difficult to envisage whether epidemiological studies that are possible in humans, would ever be able to demonstrate the link without a direct biomarker for the MIE and KE2. Available experimental models (Sanjuan-Pla et al 2015) are in conformation with this AOP, except that in the experimental in vivo models, a very protracted appearance of leukaemia is not in line with a very short latency of infant leukaemia in human.
It is obvious that there exists a vast gap between wide exposure to potential Topo II poisons and the rarity of infant leukaemia. On the basis of studies in human adult and childhood leukemias, there are a large number of genetic, epigenetic and host factors potentially modifying the link between Topo II poisons and leukaemia. Because of the rarity of the disease, it is difficult to envisage an even partial proofing these factors as of importance for the infant leukaemia.
Response-Response and Temporality Concordance for the model compound etoposide
|
Concentration of etoposide |
KE1 DNA DSB consequent to topo II inhibition |
KE2 MLL chromosomal rearrangement |
AO Infant leukaemia |
|
0.01 – 0.1 µM, in vitro(TopII enzymes and cells in culture) |
+++ (DNA damage response in various cells) |
- |
|
|
0.1 – 1 µM, in vitro cell cultures |
+++ (haematopoietic progenitor and stem cells) |
+ |
|
|
0.5-5 µM, ex vivo, mouse fetal liver HSC concentration1 |
+++ (inference from MLL cleavage) |
+ (only MLL cleavage) |
- (no leukemia development) |
|
max 5 µM, ex vivo, mouse fetal liver HSC concentration1 |
+++ (inference from MLL cleavage) |
+ MLL fusions detected only in DNA repair deficient mice |
- (no leukemia development) |
|
Max >150 µM, plasma concs in etoposide-treated patients2 |
+++ (inference from MLL cleavage) |
++ MLL-AF43 fusion gene and protein |
+ treatment-related acute leukaemia |
1a range of concentrations is linearly extrapolated on the basis of the concentration of 5 µM after the dose of 10 mg/kg.
2plasma concentration of etoposide cannot be directly extrapolated to the concentration at the active site. Probably the actual active cellular concentrations of etoposide is much lower, perhaps 10 % or less of the plasma concentration.
3 MLL-AF4: Mixed lineage leukaemia gene with chromosomal translocation t(4;11)(q21;q23)
3. Strength, consistency of the experimental evidence, and specificity of association of AO and MIE
Chapter1.
Regarding the treatment-related acute myeloid leukaemia, strength, consistency and specificity of association of AO and MIE is strong, because only etoposide have strong evidence for causing acute leukaemia in human via the general process of the AOP described here. Evidence supporting the causal relationship between etoposide-induced TopoII inhibition, DSB and the MLL rearrangement leading to the fusion gene is also strong regarding treatment-related acute leukaemia. However, the evidence as such is indirect as it is occuring in an adult population and not following in-utero exposure and consequently; therefore, lacking a model able to reproduce the full IFL disease pattern, the overall biological plausibility is considered moderate and the empirical support is also moderate. The bioflavonoid-rich diet in pregnant women has been suggested to initiate infant leukaemia by an analogous causality between inhibition of TopoII enzymes in the target sensitive cells i.e. HSPC and creation of the fusion gene. However, there is no direct evidence in humans and it is also difficult or impossible to study. Power of epidemiological studies is relatively weak in the case of a very rare disease and case-control or spatio-temporal cluster studies have barely suggested a causal relationship between exposures and disease. Although the empirical support for the chemical stressor etoposide and the metabolite etoposide quinone should be considered strong, this still remains a limitation for the overall strenght of the weight of evidence for the empirical support. However, the biological plausibility linking topoII poisons to MLL rearrangements, when occuring in the appropiate cell population ie. prehematopoietic stem cell, is strong. Although direct observations on the initial MIE in infant leukaemia are not possible, there is a lot of inferential evidence from animal and in vitro cellular studies suggesting that infant leukaemia recapitulates, at least at an apparent process level, the treatment-related adult leukaemia. It is important to recognize that in the therapy-related AML this has been clearly demonstrated with abnormalities affecting MLL locus. Chlorpyrifos is reported to be a Topo II poison and to induce MLL translocation in the human liver haematopoietic stem cells (Lu et al. 2015). However, it is probable that the dose dependence of the formation of DSBs and fusion genes is linear only in a very restricted “window” of dose range. In addition, the effect described by Lu et al. 2015 (induction of MLL translocations through caspase 3-dependent genomic instability and Topoisomerase II inhibition in human fetal liver hematopoietic stem cells) was not reproduced by Rodriguez et al. 2020. Considering the rarity of IFL and the common exposure to Topo II poisons like bioflavonoids, specificity is therefore low. However, this consideration is limited by lack of experimental studies conducted with other than anticancer drugs on the sensitive target cells .e. the liver haematopoietic stem cell. Exposure to etoposide is directly linked to DSB, which is directly linked to MLL translocation, which is only indirectly linked to secondary myeloid leukemia in cancer patients; as the MIE and first two KEs have more empirical evidence to support their relationship. There is also empirical evidence that exposure to topo II inhibitors increases the incidence of MLL. The strengths of these relationships would not be diminished by the fact that what happens once the MLL is formed would be more complicated and less established if the MLL is occurring in the foetus.
Domain of Applicability
DNA topoisomerases are key ubiquitous enzymes at all levels of living organisms. Important differences in sensitivity to topoisomerases inhibition might exist among different cell types and hematopoietic stem and progenitor cells can be a sensitive target during a critical developmental period. Foetuses and newborns show that both the baseline and chemically induced micronuclei frequencies are higher in the foetus and infant than in adults.
The available evidence do not allow for evaluating whether any significant difference occurs among cell types or species in regard to the KE event " MLL chromosomal translocation". Fetal liver hematopoietic stem cells are more susceptible to the model chemical etoposide than maternal bone marrow mononuclear cells and this has been also observed in mouse.
The AO "infant lekaemia" is a pediatric leukaemia and in animals the disease is not known and the artificial reproduction of the disease in animal models have limitations.
Essentiality of the Key Events
In line with the defining question, essentiality for this AOP is moderate. However, the actual knowledge of the IFL is supporting the evidence that IFL is a “single hit” developmental disease and MLL translocation is an essential KE based on the probability linking MLL translocation and the occurrence of the disease. Based on this the overall essentiality can be considered moderate to strong.
Essentiality of the KEs; WoE analysis
|
Support for Essentiality of KEs |
Defining Question Are downstream KEs and/or the AO prevented if an upstream KE is blocked? |
High (Strong) |
Moderate |
Low(Weak) |
|
Direct evidence from specifically designed experimental studies illustrating essentiality for at least one of the important KEs (e.g. stop/reversibility studies, antagonism, knock out models, etc.) |
Indirect evidence that sufficient modification of an expected modulating factor attenuates or augments a KE leading to increase in KE down or AO |
No or contradictory experimental evidence of the essentiality of any of the KEs |
||
|
MIE Binding to (interfers with) topoisomerase II enzyme |
MODERATE |
Although there are no direct experimental studies to demonstrate that blocking action of TopoII poisons would prevent the AOP, there are considerable evidence for the relationship between the concentration of etoposide and the formation of the MLL rearrangements in human (pre)haematopoietic progenitor/stem cells, which strongly suggest the essentiality of TopoII inhibition (e.g. Bueno et al 2009; Nanya et al 2015). In addition, chemical-induced DNA breakpoints are associated with predicted Topo II cleavage sites (ie MLL), supporting an essential role for TOPO II mediate breakage (Hernandez and Menendez 2016; Montecucco et al 2015). In human patients, therapy-related acute leukaemia characterized by MLL rearrangement is predominantly associated with etoposide treatment (Super et al. 1993) |
||
|
KE1 DNA-DSB |
STRONG | Topisomerases are nuclear enzymes taht play essential role in DNA replication, transcription, chromosome segregation and recombination. All cells have the type I and type II enzymes. Etoposide, a Topo II inhibitor, kills cells by inhibiting the enzyme to ligate DNA (Smith 2014), which leads to the accumulation of DNA-DSBs. DNA-DSBs are indeed critical lesions resulting in a wide variety of genetic alterations including traslocations (Shirvastav 2008). Persistent or incorrectely repaired DSBs can results in chromosome loss, deletion, translocation, or fusion, which can lead to carcinogenesis (Raynard 2017) | ||
|
KE2 MLL chromosomal translocation |
MODERATE. |
Growing scientific evidence, including the stable genome of the patients, suggests that infant leukaemia originates from one “big-hit” occurring during a critical developmental window of stem cell vulnerability (Andersson et al 2013; Sanjuan-Pla et al 2015; Greaves 2015). Therefore, the totality of evidence suggests the essential role of the formation of MLL-partner fusion gene and product in causing pleiotropic effects in the affected cell and directing it to the obligatory pathway to the adverse outcome of Infant leukaemia. The MLL-AF4 fusion gene is present in bone marrow mesenchymal stem cells in infant leukaemia patients, but not in patients of childhood leukaemia, suggesting that the origin of the fusion gene is probably prehaematopoietic and essential for development of IFL (Menendez et al 2009). TopoII ‘poisons’ etoposide and bioflavonoids (and some other chemicals) promote MLL rearrangements in in vitro prenatal cells or in utero. There are in vitro cellular and n vivo xenograph studies demonstrating that upon inhibiting signalling pathways from the fusion product on, cells can resume differentiation or clonal expansion of fusion gene-carrying cells is prevented (Benito et al 2015; Buechele et al 2015; Chen and Armstrong 2015). However, in absence of a relevant in vivo experimental model these findings are suggestive but not yet totally convincing. Many fusion protein have been shown to recruit disruptor of telomeric silencing 1-like (DOT1L). Although DOT1L is not genetically altered in the disease per se its mislocated enzymatic activity is a direct consequence of the chromosomal translocation. The enzymatic activity of DOT1L is critical to the occurence of MLL because methyltransferases-deficient DOT1L is capable of suppressinggrowth of MLL rearranged cells. A small-molecule inhibitor of DOT-1L inhibits cellular H3K79 methylation, blocks leukaemogenic gene expression, and selectivity kills cultured cells bearing MLL-translocation (Chen and Amstrong 2015). Animal models expressing MLL-AF4 fusion gene exist(Chen et al., 2006; Metzler et al., 2006; Krivtsov et al., 2008; Bursen et al., 2008; Tamai et al., 2011) . Leukaemia is ultimately developed the models though latency is protracted (Sanjuan-Pla et al., 2015). Expression of the MLL-AF4 (or its reciprocal) fusion gene in these models is capable of triggering leukaemia, but it is unknown whether facilitating or additional changes are required during the long latency in the mouse. The MLL-AF4 knock-in mouse developed leukaemia only after a prolonged latency (Chen et al., 2006), thus not recapitulating the ‘pathognomonic’ feature of infant leukaemia. Other animal models have been developed with similar results (see Sanjuan-Pla et al., 2015). Lin et al. (2016) designed a fusion gene between human MLL and murine af4 and demonstrated that it could transform–via retroviral transduction–human CD34+ cells to generate pro-B-ALL with all the characteristic features of the MLL-AF4 infant leukaemia. |
||
Evidence Assessment
Biological plausibility.
The biological plausibility for this AOP is overall moderate. The relationship between Topo II inhibition, DNA double strand breaks, MLL chromosomal translocation and infant leukaemia is well established. Although this pathway is reproducible in chemotherapy-induced acute myelod leukaemia in patients following treatment with etoposide, a known Topo II poison, direct evidence for IFL is not available
|
1 Support for Biological Plausibility of KERs |
Defining Question |
High (Strong) |
Moderate | low (weak) |
|
Is there a mechanistic (i.e. structural or functional) relationship between KEup and KE down consistent with established biological knowledge? |
Extensive understanding of the KER based on extensive previous documentation and broad acceptance. |
The KER is plausible based on analogy to accepted biological relationship, but scientific understanding is not completely established. | There is empirical support for a statistical association between KES but the structural or functional relationship between them is not understood. | |
|
MIE to KE1 Binding to (interfers with) topoisomerase II enzymes, leads to DNA-DSB |
STRONG |
Rationale: Although type II topoisomerases are essential to cell proliferation and survival, they have a significant genotoxic potential consequent to the resulting (double) strand breaks following enzymes inhibition. Mis-repair of accumulated of DNA double strand breaks can result in chromosomal translocations which can persist in survived cells (Mc Clendon et al. 2009, Raynard 2017). |
||
|
KE1 to KE2 DNA-DSB leads to MLL chromosomal translocation |
STRONG |
Rationale: Studies on identical twins and neonatal blood samples support the biological plausibility of this KER (Sanjuan-Pla et al 2015). Furthermore, a study in pregnant mice demonstrates that exposure of the foetus to etoposide causes DNA-DSB and MLL chromosomal translocation analogous to the human translocation except the principal fusion partner (Nanya et al 2015). Evidence from human prehaematopoietic/mesenchymal stem cells and foetal liver haematopoietic progenitor and stem cells strengthen the plausibility. Experimental evidence in these cell lines has demonstrated that etoposide, as a TopII poison, causes DSBs in MLL and partner genes, which leads to the formation of fusion genes and their products (SanjuanPla et al 2015). MLL translocation sites (breakpoint sequences) in the therapy-related leukaemia fall within a few base pairs of etoposide-induced enzyme-mediated DNA cleavage site. Although rearrangements associated with infant leukaemias are often more complex than those observed in treatment-related leukaemias, many are nevertheless associated with stable TopII-mediated DNA cut sites (Cowell and Austin 2012; Pendleton et al 2014) |
||
|
KE2 to AO MLL chromosomal translocation leads to Infant leukaemia |
MODERATE |
Rationale: The basic processes underlying overt leukaemia development are well understood and accepted. There is a general understanding of the molecular and epigenetic mechanisms leading to differentiation blockage and clonal expansion and there is evidence that the principal MLL-fusion genes and proteins harbour the necessary properties to execute the pathways associated with differentiation blockage and clonal expansion (Benito et al 2015; Chen and Armstrong 2015; Chen et al 2015).However, due to the complexity of the AO, the biological plausible link can only be indirectly established using the cancer therapy acute leukaemia following treatment with etoposide as a model. |
||
Empirical support
The overall empirical support, using the chemical tool etoposide, is moderate. In vivo and, mainly in-vitro, experiments exist but they are lacking a clear dose or concentration response relationship. In addition, only one chemical, etoposide, is considered for the empirical support.
|
3 Empirical support for KERs |
Defining Question Does the empirical evidence support that a change in the KEup leads to an appropriate change in the KE down? Does KEup occur at lower doses and earlier time points than KE down and is the incidence of KEup higher than that for KE down? Are inconsistencies in empirical support cross taxa, species and stressors that don’t align with expected pattern of hypothesized AOP? |
High (Strong) |
Moderate |
Low(Weak) |
|
Multiple studies showing dependent change in both exposure to a wide range of specific stressors (extensive evidence for temporal, dose-response and incidence concordance) and no or few critical data gaps or conflicting data. |
Demonstrated dependent change in both events following exposure to a small number of specific stressors and some evidence inconsistent with expected pattern that can be explained by factors such as experimental design, technical considerations, differences among laboratories, etc. |
Limited or no studies reporting dependent change in both events following exposure to a specific stressor (ie endpoints never measured in the same study or not at all); and/or significant inconsistencies in empirical support across taxa and species that don’t align with expected pattern for hypothesized AOP |
||
|
MIE to KE 1 Binding to (interfers with) topoisomerase II leads to DNA DSB. |
STRONG |
Rationale: Experimental evidence in pre-hematopoietic/mesenchymal cell lines has demonstrated that etoposide as a TopII poison causes DSBs in MLL and partner genes, which leads to the formation of fusion genes and their products (SanjuanPla et al 2015). In standard eukariotic cell models, production of DNA DSB is the expected outcome following treatment with Topo II poisons chemoterapeutic or antibiotic agents. |
||
|
KE1 to KE2 DNA-DSB leads to MLL chromosomal translocation |
MODERATE |
Rationale: Evidence comes from in vitro studies in appropriate human cells and from an in vivo/ex vivo study in pregnant mice; the stressor has been etoposide in most of the experiments (Libura et al 2005; Whitmarsh et al 2003; Lovett et al 201, Nanya et al 2015). Some evidence to back this KER comes from in vitro studies with bioflavonoids, especially quercetin, genistein and kaempferol (Barjesteh et al 2007). |
||
|
KE2 to AO MLL chromosomal translocation leads to Infant leukaemia |
MODERATE |
Rationale: There are a number of factors and pathways linking the fusion products with differentiation blockage and clonal expansion (Marschalek 2010; Sanjuan-Pla et al 2015). MLL encodes a protein homologous to the Drosophila trithorax gene, which has relevant functions in embryogenesis and haematopoiesis (Ernest et al 2004, Hess et al 1997). Studies with MLL-AF4, MLL-AF9 (t(9;11)(p22;q23)) and MLL-ENL (t(11;19)(q23;p13.3)) (Barabe et al 2007; Mulloy et al 2008) have clearly demonstrated how MLL chromosomal rearrangements block differentiation and enhance clonal expansion There are several animal models, in which MLL-rearranged fusion genes have been expressed and leukaemia developed (Chen et al 2006; Metzler et al 006; Krivtsov et al 2008; Bursen et al 2008; Tamai et al 2011). Engineered human hematopoietic stem and progenitor cell carrying an MLL rearrangement showed that a subset of cells persisted over time and demonstrated a higher clonogenic potential in colony forming assay (Breese et al. 2015). Cells engineered to carry MLL-AF9 and MLL-ENL fusions demonstrated leukaemogenicity especially after ex vivo and repeated transplantation (Buechele et al 2015). |
||
Uncertainties and Inconsistencies
- Evidence of the MIE is difficult to obtain in humans and one has to resort to in vitro cellular systems, which may be inadequate to take into consideration the potential effects of microenvironments, rapidly changing developmental stages and facilitating and modifying factors like the one occuring in early phase of development
-
A prerequisite for the specific outcome, i.e. creation of chromosomal rearrangement, is that TopoII inhibition has to occur in an especially vulnerable and correct hot spot in the MLL locus; however, details of this process and how it happens are not clear.
-
Etoposide does induce a large number of MLL rearrangements, most of them occur within non-coding regions, therefore not eliciting any direct oncogenic consequence. A MLL-AF4 in frame fusion is a rare event that needs to occur in a target cell within a relatively small and spatially restricted cell population during the appropriate, epigenetically plastic, developmental window; thus it may be difficult to empirically support this process.
-
Dose-response relationships between etoposide and treatment-related leukaemia are difficult to unravel, but risk of leukaemia seems to increase with larger total exposure to etoposide. However, comparison of exposures or kinetics of etoposide between leukaemia patients and non-leukemic treated subjects did not reveal any significant differences (Relling et al 1998). Also, it is not known whether the etoposide (or metabolite) concentrations during the treatment are of significance. In child and adult chemotherapy, concentrations are extremely variable between individuals; the lowest through plasma concentrations of etoposide have been of the order of 1 µM and peak concentrations very much higher. For example, in a study of Relling et al (1998), the maximum plasma concentration of etoposide was about 90 µM and that of etoposide catechol about 100-times less, below 1 µM. In another high dose chemotherapy study (Stremetzne et al 1997), the etoposide concentration was 170 µM and that of the catechol metabolite 5.8 µM maximally. However, it is not straightforward to juxtapose plasma concentrations and the tissue or cell concentration which TopoII enzyme ’sees’. Penetration of etoposide or its metabolite through plasma membrane is probably rather slow and it has been shown that the brain cancer tissue (metastasis or glioma) to plasma ratio for etoposide is only 0.1 (Pitz et al 2011). Blood-brain barrier is not necessarily a good model for cross-membrane distribution, but may give some idea about the general distributional behaviour of a drug. Even if the active target concentration of etoposide is only 10 % of the plasma concentration, it is still in the same range as the effective concentrations in cellular studies (see above). A final note on relevant concentrations: etoposide concentrations resulting in DSB and fusion gene are probably within a relatively restricted range. The concentration resulting in a proper fusion gene should be in a range which gives rise to a partially repaired insult and cells bypassing death and accumulating the abnormality.
- Animal models are a possibility (e.g. Nanya et al 2015), but are naturally prone to species-specific factors.
- An important problem is to provide a convincing and experimentally justified explanation for the dilemma between the rarity of disease in the face of pervasive exposure to topoII inhibitors.
- The treatment-related AML apparently is a true surrogate for the infant leukaemia, at least mechanistically. Is it only because of etoposide as a principal chemical intiator has provided many crucial findings for understanding the infant leukaemia.
- The ‘poisoning’ of the TopoII-DNA cleavage complex has not been shown in the putative target cell, which is still not unequivocally identified.
- MLL-AF4 knock-in mice develop leukaemia only after a prolonged latency (e.g. Chen et al 2006), thus not recapitulating the ‘pathognomonic’ feature of infant leukaemia.
- The inability of available in vivo models to recapitulate the whole AOP process is due to a crucial factor which has not yet been found, or to model-specific peculiarities.
- In the face of the rarity of the disease, epidemiological studies especially concerning aetiology and risk factors are not powerful enough to provide robust answers. For instance, investigating the hypothesized relationship of bioflavonoids with infant leukaemia will have to consider the gap between the widespread intake of these phytochemicals and the very rare occurrence of the disease.
- The biology of the disease (i.e. IFL) and the experimental studies conducted with etoposide, indicate in-utero exposure of hematopoietic stem cells (HSC) as the most critical, if not essential, factor for the development of the AO . However, a clear comparative quantification in terms of dose response vs different time of exposure and cell systems is lacking.
- The very early embryonic structure and the liver haematopoietic stem cells in particular, are representing the target cell for this AOP. A clear understanding of a higher sensitivity of HSC vs, mature hematopoietic cells, particularly in the standard genotoxicity test battery (e.g. chromosome aberration, micronucleus test, mouse lymphoma test) is lacking and more chemicals and comparative assays should be tested to scientifically validate this cell system.
- The role of fusion partners in the process of leukaemogenesis has not been completely elucidated and is representing an important uncertainty for this AOP. Normally, all of them participate in chromatin modifying complex, for example, acting on the transcriptional regulation of target genes. The MLL fusion proteins are dysregulating this highly regulated process and probably different fusion partners are working in a distinct way with variable modulatory effect on signalling pathways in leukaemic cells. Recruitment of DOT1L or officially KMT4, a histone methyltransferase, seems to be a common feature of many oncogenic MLL fusion proteins, resulting in the over methylation and overexpression of several MLL target genes encoding for transcription factors involved in body patterning and hematopoiesis. It is indeed possible that an additional (epi)genetic KE would occur downstream to MLLtranslocation, but a better understanding of the role of fusion partners in the process of leukaemogenesis would be necessary before adding it and at the moment this should be considered as a knowledge gap for this AOP.
- On the basis of studies in human adult and paediatric leukaemia, there is a large number of genetic, epigenetic and host factors potentially modifying the link between various chemical exposures and leukaemia. Because of the rarity of the disease, it is difficult to envisage, even partially, aetiological factors as of importance for the infant leukaemia.
- Transcription activator-like effector nuclease (TALEN)-mediated genome editing was used to generate endogenous MLL-AF9 and MLL-ENL oncogenes in primary human HSPCs derived from human umbilical cord plasma (Buechele et al., 2015). Engineered HSPCs displayed altered in vitro growth potential and induced acute leukaemias following transplantation in immunocompromised mice at a mean latency of 16 weeks.
Known Modulating Factors
Quantitative Understanding
The WOE analysis indicates that many KEs and KERs lack especially experimental evidence, but overall the analysis supports the qualitative AOP. The strong element in the development of the qualitative AOP is the biological plausibility of the overall pathway that it can partially be based on studies in human treatment-related disease recapitulating many crucial features of the infant leukaemia. The lack of sufficient experimental data and uncertainties in quantitative information from treatment-related acute leukaemia makes it problematic to build convincing dose (concentration)-response and response-response relationships and to identify possible practical thresholds for stressors. The MIE is expected to show a dose response relationship to a certain extent. However, it is probable that the dose dependence of the formation of DSBs and fusion genes is linear only in a very restricted “window”. In too-low concentrations the outcome of the stressor is a successful repair of the break, in too-high concentrations the outcome is cell death. It should be kept in mind additionally that the quantification of dose-responses should also consider the different sensitivity of cell systems that should be also representative of the specific time-window of exposure (i.e. in-utero).
The most pressing future need is an adequate and robust experimental model system, able to recapitulate the KEs included in this AOP, for the evaluation of relationships between doses, concentrations and responses within a temporal framework of the AOP.
Considerations for Potential Applications of the AOP (optional)
Applicability of the AOP
The proposed AO is strictly life stage-dependent, being linked with in utero exposure and early embryogenesis. However, the surrogate disease (i.e. chemotherapy-related acute leukaemia) is not life stage restricted as well as the genotoxic hazard is not expected to be life stage related.
Potential regulatory applications of the AOP
This AOP was initiated with the intention to use an epidemiologically proposed human health outcome as AO and build back an AOP leading to this. Infant childhood leukaemia is a human disease and consequently apical regulatory endpoints can only explore the hazard by means of surrogate testing. These include carcinogenesis assays and blood cell analyses in the in vivo toxicology assessment. Considering the unique biology of this AO, these tests show some technical limitations and also the sensitivity and specificity of the available tests for the AO is limited. Additionally, experimental animal models replicating the AO are limited. Technical limitations of the standard regulatory tests include: Standard carcinogenesis studies do not include an early in-utero exposure time, blood cell analysis is not a standard requirement in the extended multi-generation reproductive toxicity study and no cancer-related endpoints are included in this study. In addition, considering the rarity and the complexity of the disease, the sensitivity and specificity of these tests to capture this hazard is likely to represent a big hurdle and the regulatory tests are unlikely to represent the best way to explore this AO.
This AOP is however indicating that the MIE and the KE1 can be measured in scientific validated test assays.
With these premises, the authors support the use of this AOP during the process of assessment of epidemiological studies and the use of the AOP framework to support the biological plausibility of the effects observed in the epidemiological studies when experimental and toxicological studies are indicative that the AOP is affected and this should guide on which additional studies should be performed, if the case, to integrate the AOP framework into the MOA framework for specific chemical entities.
In addition, this AOP should serve in guiding testing strategy. This include the exploration of Topo II poison characteristics of a chemical and, if the genotoxicity standard regulatory testing battery is negative, considerations should be made on the sensitivity of the cell system used in the assay (i.e.liver HSPC).
References
Andersson AK, Ma J, Wang J, et al. The landscape of somatic mutations in infant MLL-rearranged acute lymphoblastic leukemias. Nat Genet 2015 Apr;47(4):330-337. doi: 10.1038/ng.3230.
Buechele C, Breese EH, Schneidawind D, Lin CH, Jeong J, Duque-Afonso J, Wong SH, Smith KS, Negrin RS, Porteus M, Cleary ML. MLL leukemia induction by genome editing of human CD34+ hematopoietic cells. Blood 2015 Oct 1;126(14):1683-1694. doi: 10.1182/blood-2015-05-646398.
Bueno C, Catalina P, Melen GJ, Montes R, Sanchez L, Ligero G, Garcia-Perez JL, Menendez P. Etoposide induces MLL rearrangements and other chromosomal abnormalities in human embryonic stem cells. Carcinogenesis 2009; 30(9): 1628-1637. doi: 10.1093/carcin/bgp169.
Bursen A, Schwabe K, Ruster B, et al. The AF4.MLL fusion protein is capable of inducing ALL in mice without requirement of MLL.AF4. Blood. 2010;115(17):3570-3579.
Chen C-W, Armstrong SA. Targeting DOT1L and HOX gene expression in MLL-rearranged leukemia and beyond. Exp Hematol 2015; 43: 673-684.
Chen W, Li Q, Hudson WA, Kumar A, Kirchhof N, Kersey JH. A murine Mll-AF4 knock-in model results in lymphoid and myeloid deregulation and hematologic malignancy. Blood. 2006; 108(2):669–77. doi: 10.1182/blood-2005-08-3498
Dobbins SE1, Sherborne AL, Ma YP, Bardini M, Biondi A, Cazzaniga G, Lloyd A, Chubb D, Greaves MF, Houlston RS. The silent mutational landscape of infant MLL-AF4 pro-B acute lymphoblastic leukemia. Genes Chromosomes Cancer 2013 Oct;52(10):954-60. doi: 10.1002/gcc.22090. Epub 2013 Jul 26.
Ferreira JD, Couto AC, Pombo-de-Oliveira MS, Koifman S; Brazilian Collaborative Study Group of Infant Acute Leukemia. In utero pesticide exposure and leukemia in Brazilian children < 2 years of age. Environ Health Perspect 2013 Feb;121(2):269-75. doi: 10.1289/ehp.1103942.
Ford AM, Ridge SA, Cabrera ME, Mahmoud H, Steel CM, Chan LC, et al. In utero rearrangements in the trithorax-related oncogene in infant leukaemias. Nature. 1993;363(6427):358–60. Epub 1993/05/27. pmid:8497319.
Gale KB, Ford AM, Repp R, Borkhardt A, Keller C, Eden OB, et al. Backtracking leukemia to birth: identification of clonotypic gene fusion sequences in neonatal blood spots. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(25):13950–4. Epub 1998/02/12. pmid:9391133; PubMed Central PMCID: PMC28413.
Greaves M. When one mutation is all it takes. Cancer Cell 2015; 27(4): 433-434.
Jansen MW, Corral L, van der Velden VH, Panzer-Grumayer R, Schrappe M, Schrauder A et al. Immunobiological diversity in infant acute lymphoblastic leukemiais related to the occurence and type of MLL rearrangment. Leukemia 2007; 21(4): 633-641.
Joannides M, Grimwade D. Molecular biology of therapy-related leukaemias. Clin Transl Oncol 2010 Jan;12(1):8-14. doi: 10.1007/s12094-010-0460-5.
Joannides M, Mays AN, Mistry AR, Hasan SK, Reiter A, Wiemels JL, Felix CA, Coco FL, Osheroff N, Solomon E, Grimwade D. Molecular pathogenesis of secondary acute promyelocytic leukemia. Mediterr J Hematol Infect Dis 2011;3(1):e2011045. doi: 10.4084/MJHID.2011.045.
Krivtsov AV, Feng Z, Lemieux ME, et al. H3K79 methylation profiles define murine and human MLL-AF4 leukemias. Cancer Cell. 2008;14(5): 355-368.
Lin S, Luo RT, Ptasinska A, Kerry J, Assi SA, Wunderlich M, Imamura T, Kaberlein JJ, Rayes A, Althoff MJ, Anastasi J, O'Brien MM, Meetei AR, Milne TA, Bonifer C, Mulloy JC, Thirman MJ. Instructive Role of MLL-Fusion Proteins Revealed by a Model of t(4;11) Pro-B Acute Lymphoblastic Leukemia. Cancer Cell. 2016 Nov 14;30(5):737-749. doi: 10.1016/j.ccell.2016.10.008.
Menendez P, Catalina P, Rodriguez R, Melen GJ, Bueno C, Arriero M, Garcia-Sanchez F, Lassaletta A, Garcia-Sanz R, Garcia-Castro J. Bone marrow mesenchymal stem cells from infants with MLL-AF4+ acute leukemia harbor and express the MLL-AF4 fusion gene. J Exp Med 2009 Dec 21;206(13):3131-41. doi: 10.1084/jem.20091050.
Metzler M, Forster A, Pannell R, et al. A conditional model of MLL-AF4 B-cell tumourigenesis using invertor technology. Oncogene. 2006;25(22):3093-3103.
Mondrala S, Eastmond DA. Topoisomerase II inhibition by the bioactivated benzene metabolite hydroquinone involves multiple mechanisms. Chem Biol Interact. 2010 Mar 19;184(1-2):259-68. doi: 10.1016/j.cbi.2009.12.023. Epub 2009 Dec 23. PMID: 20034485.
Nanya M,Masaki Sato,Kousuke Tanimoto,Minoru Tozuka,Shuki Mizutani ,Masatoshi Takagi .Published: December 11, 2015 https://doi.org/10.1371/journal.pone.0144540. Dysregulation of the DNA damage response and KMT2A rearrangment in fetal liver hematopoietic cells.
Pombo-de-Oliveira MS, Koifman S; Brazilian Collaborative Study Group of Infant Acute Leukemia. Infant acute leukemia and maternal exposures during pregnancy. Cancer Epidemiol Biomarkers Prev 2006 Dec;15(12):2336-41.
Raynard S., Niu H., Sung P. 2017. DNA double-strand break processing: the biginning of the end. genes & development 22: 2903-2907.
Relling MV, Yanishevski Y, Nemec J, Evans WE, Boyett JM, Behm FG, Pui CH.Etoposide and antimetabolite pharmacology in patients who develop secondary acute myeloid leukemia. Leukemia. 1998 Mar;12(3):346-52.
Rodríguez‐Cortez, V C, Menéndez, P, 2020. Genotoxicity of permethrin and clorpyriphos on human stem and progenitor cells at different ontogeny stages: implications in leukaemia development. EFSA supporting publication 2020: 17( 5): EN‐1866. 35 pp. doi: 10.2903/sp.efsa.2020.EN‐18
Sam TN, Kersey JH, Linabery AM, Johnson KJ, Heerema NA, Hilden JM, et al. MLL gene rearrangements in infant leukaemia vary with age at diagnosis and selected demographic factors: a Children’s Oncology Group (COG) study. Pediatr Blood cancer. 2012; 58 (6): 836-839.
Sanjuan-Pla A, Bueno C, Prieto C, Acha P, Stam RW, Marschalek R, Menendez P. Revisiting the biology of infant t(4;11)/MLL-AF4+ B-cell acute lymphoblastic leukemia. Blood 2015; 126(25): 2676-2685 DOI 10.1182/blood-2015-09-667378.
Smith NA, Byl LAW, Mercer SL, Deweese JE and Osheroff N. 2014. Etoposide quinone is a covalent poison of human topoisomerase II beta. biochemistry. 53, 3229-3236.
Stremetzne S, Jaehde U, Kasper R, Beyer J, Siegert W, Schunack W. Considerable plasma levels of a cytotoxic etoposide metabolite in patients undergoing high-dose chemotherapy. European Journal of Cancer 1997 May;33(6):978-9.
Tamai H, Miyake K, Takatori M, Miyake N, Yamaguchi H, Dan K, Shimada T, Inokuchi K. Activated K-Ras protein accelerates human MLL/AF4-induced leukemo-lymphomogenicity in a transgenic mouse model. Leukemia. 2011 May;25(5):888-91. doi: 10.1038/leu.2011.15.