
The authors have designated this AOP as all rights reserved. Re-use in any form requires advanced permission from the authors.
AOP: 209
Title
Perturbation of cholesterol and glutathione homeostasis leading to hepatotoxicity: Integrated multi-OMICS approach for building AOP
Short name
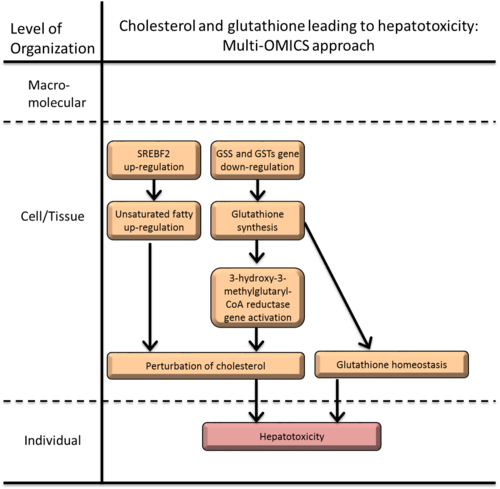
Graphical Representation
Point of Contact
Contributors
- Jinhee Choi
Coaches
OECD Information Table
| OECD Project # | OECD Status | Reviewer's Reports | Journal-format Article | OECD iLibrary Published Version |
|---|---|---|---|---|
This AOP was last modified on April 29, 2023 16:02
Revision dates for related pages
| Page | Revision Date/Time |
|---|---|
| Up Regulation, SREBF2 | September 16, 2017 10:17 |
| Up Regulation, Unsaturated fatty acid | September 16, 2017 10:17 |
| Down Regulation, GSS and GSTs gene | September 16, 2017 10:17 |
| Glutathione synthesis | September 16, 2017 10:17 |
| Activation, 3-hydroxy-3-methylglutaryl-CoA reductase gene | September 16, 2017 10:17 |
| Perturbation of cholesterol | September 16, 2017 10:17 |
| Glutathione homeostasis | September 16, 2017 10:17 |
| Hepatotoxicity | February 15, 2017 04:11 |
| Up Regulation, SREBF2 leads to Up Regulation, Unsaturated fatty acid | February 15, 2017 04:11 |
| Up Regulation, Unsaturated fatty acid leads to Perturbation of cholesterol | February 15, 2017 04:12 |
| Down Regulation, GSS and GSTs gene leads to Glutathione synthesis | February 15, 2017 04:12 |
| Glutathione synthesis leads to Activation, 3-hydroxy-3-methylglutaryl-CoA reductase gene | February 15, 2017 04:12 |
| Activation, 3-hydroxy-3-methylglutaryl-CoA reductase gene leads to Perturbation of cholesterol | February 15, 2017 04:13 |
| Down Regulation, GSS and GSTs gene leads to Glutathione homeostasis | February 15, 2017 04:13 |
| Perturbation of cholesterol leads to Hepatotoxicity | February 15, 2017 04:13 |
| Glutathione homeostasis leads to Hepatotoxicity | February 15, 2017 04:13 |
| Silica nanoparticles | February 15, 2017 04:01 |
Abstract
The system toxicology approach using ‘multi-OMICS-profiling-techniques’ (transcriptomics, proteomics and metabolomics) have proven to be robust tool for unraveling complex molecular machinery underlying various physiological, as well as, pathophysiological processes and have been successfully utilized in various fields including stress biology and toxicology. The application of multi-OMICS approaches are gaining acceptance in the regulatory community to provide information on a chemical’s hazard and risks though their mode-of-action (MOA), however, its utility in a AOP building context are still limited. This AOP is constituted the MIE as sterol regulatory element binding transcription factor 2 (SREBF2) gene activation and repressions of glutathione synthetase (GSS) and glutathione S-transferases (GSTA1, GSTA2, GSTA3, GSTA5) genes and the KEs as perturbation of cholesterol, though up regulated unsaturated fatty acid concentration, and glutathione homeostasis leading to oxidative stress, DNA damage and AO as hepatotoxicity (HepG2 cell death).
AOP Development Strategy
Context
Strategy
Summary of the AOP
Events:
Molecular Initiating Events (MIE)
Key Events (KE)
Adverse Outcomes (AO)
| Type | Event ID | Title | Short name |
|---|
| KE | 1284 | Up Regulation, SREBF2 | Up Regulation, SREBF2 |
| KE | 1285 | Up Regulation, Unsaturated fatty acid | Up Regulation, Unsaturated fatty acid |
| KE | 1286 | Down Regulation, GSS and GSTs gene | Down Regulation, GSS and GSTs gene |
| KE | 1287 | Glutathione synthesis | Glutathione synthesis |
| KE | 1288 | Activation, 3-hydroxy-3-methylglutaryl-CoA reductase gene | Activation, 3-hydroxy-3-methylglutaryl-CoA reductase gene |
| KE | 1289 | Perturbation of cholesterol | Perturbation of cholesterol |
| KE | 1290 | Glutathione homeostasis | Glutathione homeostasis |
| AO | 1291 | Hepatotoxicity | Hepatotoxicity |
Relationships Between Two Key Events (Including MIEs and AOs)
| Title | Adjacency | Evidence | Quantitative Understanding |
|---|
Network View
Prototypical Stressors
| Name |
|---|
| Silica nanoparticles |
Life Stage Applicability
| Life stage | Evidence |
|---|---|
| All life stages |
Taxonomic Applicability
Sex Applicability
| Sex | Evidence |
|---|---|
| Unspecific |