
This AOP is licensed under the BY-SA license. This license allows reusers to distribute, remix, adapt, and build upon the material in any medium or format, so long as attribution is given to the creator. The license allows for commercial use. If you remix, adapt, or build upon the material, you must license the modified material under identical terms.
AOP: 623
Title
Activation, estrogen receptor alpha leads to persistent vaginal cornification via increased kisspeptin release
Short name
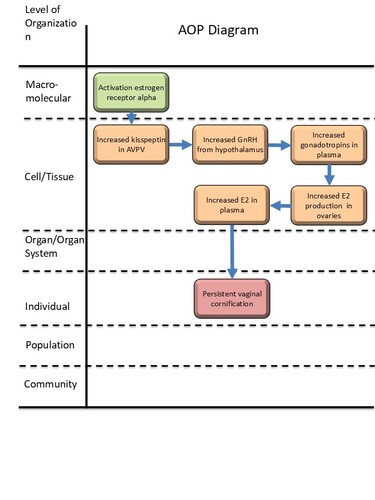
Graphical Representation
Point of Contact
Contributors
- John Frisch
Coaches
OECD Information Table
| OECD Project # | OECD Status | Reviewer's Reports | Journal-format Article | OECD iLibrary Published Version |
|---|---|---|---|---|
This AOP was last modified on February 03, 2026 11:02
Revision dates for related pages
| Page | Revision Date/Time |
|---|---|
| Activation, estrogen receptor alpha | January 28, 2026 14:32 |
| Increased Kisspeptin levels in anteroventral periventricular nucleus (AVPV) | January 29, 2026 16:57 |
| Increased, secretion of GnRH from hypothalamus | January 29, 2026 17:03 |
| Increase, Gonadotropins concentration in plasma | January 29, 2026 17:18 |
| Increased, estradiol (E2) production in ovaries | January 29, 2026 16:27 |
| Plasma estradiol, increased | January 29, 2026 17:31 |
| Persistent vaginal cornification | January 29, 2026 17:33 |
| Activation, ERα leads to Increased Kisspeptin levels in AVPV | February 12, 2026 09:13 |
| Increased Kisspeptin levels in AVPV leads to Increased, secretion of GnRH from hypothalamus | February 10, 2026 15:13 |
| Increased, secretion of GnRH from hypothalamus leads to Increase, Gonadotropins concentration in plasma | February 03, 2026 11:22 |
| Increase, Gonadotropins concentration in plasma leads to Increased, E2 production in ovaries | February 03, 2026 11:43 |
| Increased, E2 production in ovaries leads to Plasma E2, increased | February 03, 2026 11:44 |
| Plasma E2, increased leads to Persistent vaginal cornification | February 03, 2026 11:49 |
Abstract
Estrogen receptor alpha (ERa) is a nuclear transcription factor involved in regulation of many physiological processes in mammals. Binding by estrogen induces the transcription of target genes. Here we focus on the role of ERa in the hypothalamus- pituitary-gonadal (HPG) axis involved in reproductive development and puberty through activation of kisspeptin. For an overview of the role of various hormones in initiating puberty, as well as some common disorders from disruption of hormone signalling in humans, see Howard (2021).
Kisspeptin is a key signalling neuropeptide hormone in mammals. Positive feedback for kisspeptin production is due to increased levels of estrogen binding to Estrogen Receptor Alpha (ERa) receptors in neurons from the anteroventral periventricular nucleus (AVPV) region of the hypothalamus, while negative feedback for kisspeptin hormone production is due to ERa receptor activation of the neurons from the arcuate nucleus (ARC) region of the hypothalamus (Uenoyama et al. 2021). Kisspeptin signalling is important for prompting hormone production for initiating the development of reproductive organs during puberty in females.
Increased kisspeptin results in increased hypothalamic-pituitary-gonadal hormone signaling by activating increased Gonadotropin-releasing hormone (GnRH) secretion, a peptide hormone produced by the hypothalamus (Hassanein et al. 2024). Increases in GnRH stimulates increased production of gonadotropins via a G-protein, phospholipase C activation, and mitogen-activated protein kinase (MAPK) pathway activation (Hassanein et al. 2024). Luteinizing hormone (LH) and Follicle-stimulating hormone (FSH) are gonadotropins of particular interest because of their roles in regulating gonadal steroid biosynthesis, and are released from the anterior pituitary gland (Howard 2021).
Production of estradiol (E2) by the ovaries has been well-established by the two-cell, two gonadotropin model of steroid biosynthesis (for review see Drummond 2006; Kimura et al. 2007; Palermo 2007; Beevors et al. 2024). Luteinizing hormone stimulates steroid production in theca cells, while follicle-stimulating hormone stimulates steroid production in granulosa cells. Estradiol is a key signalling estrogen hormone in the hypothalamic–pituitary-gonadal (HPG) axis cueing the initiation of development of reproductive organs and puberty in females.
Puberty occurs when reproductive organs mature and hormone levels are altered to transform an individual into one capable of reproduction (for review see Laffan et al. 2018), which includes the estrus cycle in rodents (for review see Miller and Takahashi 2014; Swift et al. 2024). Vaginal cornification occurs in response to increased estradiol during estrus, and is characterized by a keratinized cell layer (Goldman et al. 2007). Persistent vaginal cornification occurs due to a disruption of the estrus cycle resulting in a prolonged estrus period, and a failure to ovulate.
This AOP links ERa activation to persistent vaginal cornification as one of the signs of the adverse outcome precocious puberty, observed in Endocrine Disruptor Screening Program (EDSP) protocol (US EPA 2011, OECD 2025).
AOP Development Strategy
Context
This AOP was part of an Environmental Protection Agency effort to develop AOPs that establish scientifically supported causal linkages between alternative endpoints measured using new approach methodologies (NAMs) and guideline apical endpoints measured in Tier 1 and Tier 2 test guidelines (U.S. EPA, 2024) employed by the Endocrine Disruptor Screening Program (EDSP). A series of key events that represent significant, measurable, milestones connecting molecular initiation to apical endpoints indicative of adversity were identified based on scientific review articles and empirical studies. Additionally, scientific evidence supporting the causal relationships between each pair of key events was assembled and evaluated. The present effort focused primarily on empirical studies with laboratory rodents and other mammals.
Strategy
The scope of the aforementioned EPA project was to develop AOP(s) relevant to apical endpoints observed in the test guidelines, based on mechanisms consistent with empirical studies. The literature used to support this AOP and its constituent pages began with the test guidelines and followed to primary, secondary, and/or tertiary works concerning the relevant underlying biology. KE and KER page creation and re-use was determined using Handbook principles where page re-use was preferred.

Summary of the AOP
Events:
Molecular Initiating Events (MIE)
Key Events (KE)
Adverse Outcomes (AO)
| Type | Event ID | Title | Short name |
|---|
| MIE | 1065 | Activation, estrogen receptor alpha | Activation, ERα |
| KE | 1985 | Increased Kisspeptin levels in anteroventral periventricular nucleus (AVPV) | Increased Kisspeptin levels in AVPV |
| KE | 1047 | Increased, secretion of GnRH from hypothalamus | Increased, secretion of GnRH from hypothalamus |
| KE | 2137 | Increase, Gonadotropins concentration in plasma | Increase, Gonadotropins concentration in plasma |
| KE | 2402 | Increased, estradiol (E2) production in ovaries | Increased, E2 production in ovaries |
| KE | 2294 | Plasma estradiol, increased | Plasma E2, increased |
| AO | 2306 | Persistent vaginal cornification | Persistent vaginal cornification |
Relationships Between Two Key Events (Including MIEs and AOs)
| Title | Adjacency | Evidence | Quantitative Understanding |
|---|
Network View
Prototypical Stressors
Life Stage Applicability
| Life stage | Evidence |
|---|---|
| Adult, reproductively mature | Moderate |
| Juvenile | Moderate |
Taxonomic Applicability
| Term | Scientific Term | Evidence | Link |
|---|---|---|---|
| mammals | mammals | Moderate | NCBI |
Sex Applicability
| Sex | Evidence |
|---|---|
| Female | High |
Overall Assessment of the AOP
|
1. Support for Biological Plausibility of Key Event Relationships: Is there a mechanistic relationship between KEup and KEdown consistent with established biological knowledge? |
|
|
Key Event Relationship (KER) |
Level of Support Strong = Extensive understanding of the KER based on extensive previous documentation and broad acceptance. Moderate = Support of the relationship based on empirical studies, with some inference of receptor activation in laboratory mammals from in vitro studies. |
|
Relationship 2665: Activation estrogen receptor alpha leads to increased AVPV kisspeptin |
Moderate support. The relationship between activation of estrogen receptor alpha and increased AVPV kisspeptin release is broadly accepted and supported among humans and laboratory mammal data. Activation of estrogen receptor alpha is often studied in vitro, with activation of estrogen receptor alpha inferred in laboratory mammal studies when downstream effects are consistent with in vitro observations. |
|
Relationship 3714: Increased AVPV kisspeptin leads to increased GnRH |
Strong support. The relationship between increased AVPV kisspeptin and increased GnRH release is broadly accepted and supported among humans and laboratory mammal data. |
|
Relationship 3715: Increased GnRH leads to increased gonadotropins |
Strong support. The relationship between increased GnRH release and increased gonadotropins is broadly accepted and supported among humans and laboratory mammal data (i.e., reflects canonical knowledge). |
|
Relationship 3716: Increased gonadotropins leads to increased estradiol production in ovaries |
Strong support. The relationship between increased gonadotropins and increased estradiol production in ovaries is broadly accepted and supported among humans and laboratory mammal data (i.e., reflects canonical knowledge). |
|
Relationship 3717: Increased estradiol production in ovaries leads to increased plasma estradiol |
Strong support. The relationship between increased estradiol production in ovaries and increased plasma estradiol is broadly accepted and supported among humans and laboratory mammal data and reflects canonical knowledge. |
|
Relationship 3718: Increased plasma estradiol leads to persistent vaginal cornification |
Strong support. The relationship between increased plasma estradiol and persistent vaginal cornification is broadly accepted and supported by laboratory mammal data. |
|
Overall |
Moderate to Strong support. Extensive understanding of the relationships between events from empirical studies from humans and laboratory mammals, with some inference of estrogen receptor alpha activation from in vitro studies when performing laboratory mammal studies. |
Domain of Applicability
Life Stage: Adult, reproductively mature and juveniles.
Sex: Applies to females.
Taxonomic: Primarily studied in humans and laboratory rodents. Plausible for most mammals due to conserved hormone pathways regulating hypothalamus-pituitary-gonadal axis processes. For vertebrates, kisspeptin and kisspeptin receptors are absent from birds; the relationship between estrogen and kisspeptin is also unclear for fish as perhaps compensatory rather than required (Sivalingam et al 2022). GnRH, gonadotropins, and estradiol production in ovaries are widespread among amphibians, reptiles, fish, birds, and mammals (Bondesson et al. 2015; Li et al. 2019; Duan and Allard 2020; Hollander-Cohen et al. 2021; Hanlon et al. 2022; Cruz-Cano et al. 2023). Persistent vaginal cornification primarily studied in laboratory rodents with an estrus cycle.
Essentiality of the Key Events
|
2. Essentiality of Key Events: Are downstream KEs and/or the AO prevented if an upstream KE is blocked? |
|
|
Key Event (KE) |
Level of Support Strong = Direct evidence from specifically designed experimental studies illustrating essentiality and direct relationship between key events. |
|
MIE 1065 Activation estrogen receptor alpha |
Strong support. Activation of estrogen receptor alpha leads to increased AVPV kisspeptin. Evidence is available from estrogen compound studies, toxicant studies and gene-knock out studies with in vitro human cell lines and intact laboratory mammals. Best evidence for essentiality for activation of estrogen receptor alpha is the increase from baseline levels of kisspeptin following addition of estrogen compounds. Activation of estrogen receptor alpha can lead to either increase or decrease of AVPV kisspeptin release depending on the stressor and age at exposure. Broadly, stressor exposure can lead to increased AVPV kisspeptin release and subsequent increased hormone levels (Adachi et al. 2007; Clarkson et al. 2008; Tomikawa et al. 2012; Wang et al. 2014), accelerating the response to hormones in the expected direction from estrogen receptor alpha activation to increased AVPV kisspeptin release. Alternatively, neonatal developmental stressor exposure can disrupt the Hypothalamic-Pituitary-Gonadal axis, decreasing AVPV kisspeptin release and subsequently decreasing hormone levels (Bateman and Patisaul 2008; Homma et al. 2009; Navarro et al. 2009; Patisaul et al. 2009; Ichimura et al. 2015a; Ichimura et al. 2015b), dampening response to hormones. |
|
KE 1985 Increased AVPV kisspeptin |
Strong support. Increased AVPV kisspeptin leads to increased GnRH in plasma. Evidence is available from estrogen compound studies, toxicant studies, gene-knock out studies, and ovariectomized animal studies. Best evidence for essentiality for increased AVPV kisspeptin is in stressor studies with observed decreased GnRH hormone levels, and restored GnRH levels from supplemental addition of kisspeptin. |
|
KE 1047 Increased GnRH |
Strong support. Increased GnRH leads to increased gonadotropins. Evidence is available from estrogen compound studies, toxicant studies, gene-knock out studies, diet studies and ovariectomized animal studies. Best evidence for essentiality for increased GnRH is in gene-knock out studies in GnRH-null animals failing to increase gonadotropin levels. |
|
KE 2137 Increased Gonadotropins |
Strong support. Increased gonadotropins lead to increased estradiol production in ovaries. Evidence is available from hormone addition studies and in vitro studies of ovarian follicles. Best evidence for essentiality for increased gonadotropins is from hormone replacement studies in which addition of LH and FSH are both required to increase the expression of enzymes in the ovary necessary for steroid biosynthesis, leading to increased estradiol levels. |
|
KE 2402 Increased estradiol production in ovaries |
Strong support. Increased estradiol production in ovaries leads to increased plasma estradiol. Evidence is available from hormone studies and toxicant studies. Best evidence for essentiality for increased estradiol production is from hormone addition studies in which enzymes in the ovary steroid biosynthesis pathway are increased, leading to increased estradiol levels in plasma. Increased plasma estradiol concentrations can also be mimicked by by birth control pills or hormone replacement therapy, which generally use closely related synthetic compounds. |
|
KE 2294 Plasma estradiol, increased |
Strong support. Increased plasma estradiol leads to persistent vaginal cornification. Evidence is available from hormone studies and toxicant studies. Best evidence for essentiality for persistent vaginal cornification is from a myriad of neonatal estrogenization studies from injection of estradiol compounds and resulting persistent vaginal cornification and abnormal estrus cycles. |
|
AO 2306 Persistent vaginal cornification |
This is the final event of the AOP. |
|
Overall |
Strong support. Direct evidence from empirical studies from laboratory mammals and human cell lines for all key events. |
Evidence Assessment
|
3. Empirical Support for Key Event Relationship: Does empirical evidence support that a change in KEup leads to an appropriate change in KEdown? |
|
|
Key Event Relationship (KER) |
Level of Support Strong = Experimental evidence from exposure to toxicant shows consistent change in both events across taxa and study conditions. |
|
Relationship 2665: Activation estrogen receptor alpha leads to increased AVPV kisspeptin |
Strong support. Activation of estrogen receptor alpha leads to increased AVPV kisspeptin. Evidence is available from estrogen compound studies, toxicant studies and gene-knock out studies. Activation of estrogen receptor alpha occurred earlier in the time-course of exposure than increased AVPV kisspeptin, and the concentrations that activated estrogen receptor alpha were equal to or lower than the concentrations that increased AVPV kisspeptin. Therefore, the data support a causal relationship. In some in vivo laboratory mammal studies, activation of estrogen receptor alpha is inferred by kisspeptin response from a stressor known to be an ERa agonist from in vitro studies. |
|
Relationship 3714: Increased AVPV kisspeptin leads to increased GnRH |
Strong support. Increased AVPV kisspeptin leads to increased GnRH. Evidence is available from estrogen compound studies, toxicant studies, gene-knock out studies, and ovariectomized animal studies. Increased AVPV kisspeptin occurred earlier in the time-course of exposure than increased GnRH, and the concentrations that increased AVPV kisspeptin were equal to or lower than the concentrations that increased GnRH. Therefore, the data support a causal relationship. |
|
Relationship 3715: Increased GnRH leads to increased gonadotropins |
Strong support. Increased GnRH leads to increased gonadotropins. Evidence is available from estrogen compound studies, toxicant studies, gene-knock out studies, diet studies and ovariectomized animal studies. Increased GnRH occurred earlier in the time-course of exposure than increased gonadotropins, and the concentrations that increased GnRH were equal to or lower than the concentrations that increased gonadotropins. Therefore, the data support a causal relationship. |
|
Relationship 3716: Increased gonadotropins leads to increased estradiol production in ovaries |
Strong support. Increased gonadotropins leads to increased estradiol production in ovaries. Evidence is available from hormone addition studies and in vitro studies of ovarian follicles. Increased gonadotropins occurred earlier in the time-course of exposure than increased estradiol production in ovaries, and the concentrations that increased gonadotropins were equal to or lower than the concentrations that increased estradiol production in ovaries. Therefore, the data support a causal relationship. |
|
Relationship 3717: Increased estradiol production in ovaries leads to increased plasma estradiol |
Strong support. Increased estradiol production in ovaries leads to increased plasma estradiol. Evidence is available from hormone studies and toxicant studies. Increased estradiol production in ovaries occurred earlier in the time-course of exposure than increased plasma estradiol, and the concentrations that increased estradiol production in ovaries were equal to or lower than the concentrations that increased plasma estradiol. Therefore, the data support a causal relationship. |
|
Relationship 3718: Increased plasma estradiol leads to persistent vaginal cornification |
Strong support. Increased plasma estradiol leads to persistent vaginal cornification. Evidence is available from hormone studies and toxicant studies. Increased plasma estradiol occurred earlier in the time-course of exposure than persistent vaginal cornification, and the concentrations that increased plasma estradiol were equal to or lower than the concentrations that led to persistent vaginal cornification. Therefore, the data support a causal relationship. |
|
Overall |
Strong support. Evidence from empirical studies, including frequent testing in laboratory mammals, shows consistent relationships between upstream and downstream events, with upstream events occurring earlier in the time-course of exposure and at equal or lower concentrations than downstream events, supporting causal relationships. |
Known Modulating Factors
| Modulating Factor (MF) | Influence or Outcome | KER(s) involved |
|---|---|---|
Quantitative Understanding
Considerations for Potential Applications of the AOP (optional)
References
Adachi S, Yamada S, Takatsu Y, Matsui H, Kinoshita M, Takase K, Sugiura H, Ohtaki T, Matsumoto H, Uenoyama Y, Tsukamura H, Inoue K, Maeda K. 2007. Involvement of anteroventral periventricular metastin/kisspeptin neurons in estrogen positive feedback action on luteinizing hormone release in female rats. Journal of Reproduction and Development 53(2): 367-378.
Bateman HL, Patisaul HB. 2008. Disrupted female reproductive physiology following neonatal exposure to phytoestrogens or estrogen specific ligands is associated with decreased GnRH activation and kisspeptin fiber density in the hypothalamus. Neurotoxicology 29(6): 988-997.
Beevors LI, Sundar S, Foster PA. 2024. Steroid metabolism and hormonal dynamics in normal and malignant ovaries. Essays in Biochemistry 68(4): 491-507. Bondesson M, Hao R, Lin CY, Williams C, Gustafsson JA. 2015. Estrogen receptor signaling during vertebrate development. Biochimica et Biophysica Acta 1849(2): 142-151.
Clarkson J, d’Anglemont de Tassigny X, Moreno AS, Colledge WH, Herbison AE. 2008. Kisspeptin–GPR54 signaling is essential for preovulatory gonadotropin-releasing hormone neuron activation and the luteinizing hormone surge. Journal of Neuroscience 28(35): 8691–8697.
Cruz-Cano NB, Sanchez-Rivera UA, Alvarez-Rodriguez C, Cardenas-Leon M, Martinez-Torres M. 2023. Sex steroid receptors in the ovarian follicles of the lizard Sceloporus torquatus. Zygote. 31(4): 386-392.
Drummond AE. 2006. The role of steroids in follicular growth. Reproductive Biology and Endocrinology 4:16.
Duan C, Allard J. 2020. Gonadotropin-releasing hormone neuron development in vertebrates. General and Comparative Endocrinology. 292: 113465.
Goldman JM, Murr AS and Cooper RL. 2007. The rodent estrous cycle: characterization of vaginal cytology and its utility in toxicological studies. Birth Defects Research (Part B) 80: 84-97.
Hanlon C, Ziezold CJ, Bedecarrats GY. 2022. The Diverse Roles of 17β-Estradiol in Non-Gonadal Tissues and Its Consequential Impact on Reproduction in Laying and Broiler Breeder Hens. Frontiers in Physiology 13: 942790.
Hassanein, E.M., Szelenyi, Z., Szenci, O. 2024. Gonadotropin-Releasing Hormone (GnRH) and Its Agonists in Bovine Reproduction I: Structure, Biosynthesis, Physiological Effects, and Its Role in Estrous Synchronization. Animals 14: 1473.
Hollander-Cohen L, Golan M, Levavi-Sivan B. 2021. Differential Regulation of Gonadotropins as Revealed by Transcriptomes of Distinct LH and FSH Cells of Fish Pituitary. International Journal of Molecular Sciences 22(12): 6478.
Homma T, Sakakibara M, Yamada S, Kinoshita M, Iwata K, Tomikawa J, Kanazawa T, Matsui H, Takatsu Y, Ohtaki T, Matsumoto H, Uenoyama Y, Maeda K, Tsukamura H. 2009. Significance of neonatal testicular sex steroids to defeminize anteroventral periventricular kisspeptin neurons and the GnRH/LH surge system in male rats. Biology of Reproduction 81(6): 1216-25.
Howard SR. 2021. Interpretation of reproductive hormones before, during and after the pubertal transition—identifying health and disordered puberty. Clinical Endocrinology 95: 702-715.
Ichimura R, Takahashi M, Morikawa T, Inoue K, Maeda J, Usuda K, Yokosuka M, Watanabe G, Yoshida M. 2015a. Prior attenuation of KiSS1/GPR54 signaling in the anteroventral periventricular nucleus is a trigger for the delayed effect induced by neonatal exposure to 17alpha-ethynylestradiol in female rats. Reproductive Toxicology 51: 145-156.
Ichimura R, Takahashi M, Morikawa T, Inoue K, Kuwata K, Usuda K, Yokosuka M, Watanabe G, Yoshida M. 2015b. The Critical Hormone-Sensitive Window for the Development of Delayed Effects Extends to 10 Days after Birth in Female Rats Postnatally Exposed to 17alpha-Ethynylestradiol. Biology of Reproduction 93(2): 32.
Kimura S, Matsumoto T, Matsuyama R, Shiina H, Sato T, Takeyama K, Kato S. 2007. Androgen receptor function in folliculogenesis and its clinical implication in premature ovarian failure. Trends in Endocrinology and Metabolism 18(5): 183-189.
Laffan, S.B., Lorraine M. Posobiec, L.M., Jenny E. Uhl, J.E., and Vidal, J.D. 2018. Species Comparison of Postnatal Development of the Female Reproductive System. Birth Defects Research 110(3): 163-189.
Li M, Sun L, Wang D. 2019. Roles of estrogens in fish sexual plasticity and sex differentiation. General and Comparative Endocrinology 277: 9-16.
Miller, B.H. and Takahashi, J.S. 2014. Central circadian control of female reproductive function. Frontiers in Endocrinology 4(1): 195.
Navarro VM, Sánchez-Garrido MA, Castellano JM, Roa J, García-Galiano D, Pineda R, Aguilar E, Pinilla L, Tena-Sempere M. 2009. Persistent impairment of hypothalamic KiSS-1 system after exposures to estrogenic compounds at critical periods of brain sex differentiation. Endocrinology. 150(5): 2359-2367.
Organisation for Economic Co-operation and Development. 2025. Test No. 443: Extended One-Generation Reproductive Toxicity Study, OECD Guidelines for the Testing of Chemicals, Section 4, OECD Publishing, Paris. https:// https://www.oecd.org/en/publications/test-no-443-extended-one-generation-reproductive-toxicity-study_9789264185371-en.html (retrieved 11 Dec 2025)Palermo R. 2007. Differential actions of FSH and LH during folliculogenesis. Reproductive BioMedicine Online 15(3): 326-337.
Patisaul HB, Todd KL, Mickens JA, Adewale HB. 2009. Impact of neonatal exposure to the ERalpha agonist PPT, bisphenol-A or phytoestrogens on hypothalamic kisspeptin fiber density in male and female rats. Neurotoxicology. 30(3): 350-357.
Sivalingam M, Ogawa S, Trudeau VL, Parhar IS. 2022. Conserved functions of hypothalamic kisspeptin in vertebrates. General and Comparative Endocrinology 317: 113973.
Swift, K.M., Gary, N.C., and Urbanczyk, P.J. 2024. On the basis of sex and sleep: the influence of the estrous cycle and sex on sleep-wake behavior. Frontiers in Neuroscience 18:1426189.
Tomikawa J, Uenoyama Y, Ozawa M, Fukanuma T, Takase K, Goto T, Abe H, Ieda N, Minabe S, Deura C, Inoue N, Sanbo M, Tomita K, Hirabayashi M, Tanaka S, Imamura T, Okamura H, Maeda K, Tsukamura H. 2012. Epigenetic regulation of Kiss1 gene expression mediating estrogen-positive feedback action in the mouse brain. Proceedings of the National Academy of Science 109(20): E1294-E1301.
Uenoyama, Y., Inoue, N., Nakamura, S., and Tsukamura, H. Kisspeptin Neurons and Estrogen–Estrogen Receptor α Signaling: Unraveling the Mystery of Steroid Feedback System Regulating Mammalian Reproduction. 2021. International Journal of Molecular Sciences 22(17): 9229.
U.S. Environmental Protection Agency. 2004. EDSP Test Guidelines and Guidance Document. https://www.epa.gov/test-guidelines-pesticides-and-toxic-substances/edsp-test-guidelines-and-guidance-document (retrieved 25 July 2025).
U.S. Environmental Protection Agency (EPA), Office of Chemical Safety and Pollution Prevention. 2011. OCSPP test guideline 890.1600: Uterotrophic assay (EPA 740-C-09-0010). https://www.epa.gov/sites/default/files/2015-07/documents/final_890.1600_uterotrophic_assay_sep_9.22.11.pdf (retrieved 11 December 2025)
Wang X, Chang F, Bai Y, Chen F, Zhang J, Chen L. 2014. Bisphenol A enhances kisspeptin neurons in anteroventral periventricular nucleus of female mice. Journal of Endocrinology 28(35): 201-213.