
The authors have designated this AOP as all rights reserved. Re-use in any form requires advanced permission from the authors.
AOP: 499
Title
Activation of MEK-ERK1/2 leads to deficits in learning and cognition via disrupted neurotransmitter release
Short name
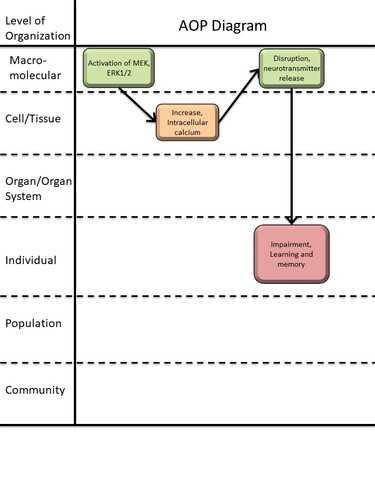
Graphical Representation
Point of Contact
Contributors
- Travis Karschnik
Coaches
OECD Information Table
| OECD Project # | OECD Status | Reviewer's Reports | Journal-format Article | OECD iLibrary Published Version |
|---|---|---|---|---|
This AOP was last modified on May 09, 2024 17:09
Revision dates for related pages
| Page | Revision Date/Time |
|---|---|
| Activation of mitogen-activated protein kinase kinase, extracellular signal-regulated kinase 1/2 | July 28, 2023 09:33 |
| Increase, intracellular calcium | July 21, 2023 16:26 |
| Impairment, Learning and memory | July 26, 2024 09:54 |
| Disruption, neurotransmitter release | July 21, 2023 16:35 |
| Activation of MEK, ERK1/2 leads to Increase, intracellular calcium | April 11, 2024 15:20 |
| Increase, intracellular calcium leads to Disruption, neurotransmitter release | April 11, 2024 15:20 |
| Disruption, neurotransmitter release leads to Impairment, Learning and memory | April 11, 2024 15:20 |
| Lead | November 29, 2016 18:42 |
| Arsenic | April 27, 2021 00:15 |
| Cadmium | October 25, 2017 08:33 |
| Manganese | February 04, 2022 14:47 |
| Heavy metals (cadmium, lead, copper, iron, nickel) | October 25, 2021 03:21 |
Abstract
Metal mixture activation of ERK1/2 and JNK1/2 in astrocytes leads to increased Ca2+ release (Asit Rai and others 2010). Alterations to calcium, an essential nutrient which is required in multiple cellular and physiological functions, such as cell adhesion, signal transduction, and neurotransmission can be expected to have downstream effects in those functions (Antonio et al., 2002). Changes in neurotransmission can then lead to changes in learning and cognitition (Neal and Guilarte 2010).
MEK-ERK1/2 is important in understanding uptake of metals into the brain and its relationship to deficits in learning and cognition from exposure to metals commonly detected at Superfund sites including lead, cadmium, manganese, and arsenic. Current risk assessment guidance dictates a largely chemical-by-chemical evaluation of exposures and risks, which fails to adequately address potential interactions with other chemicals, nonchemical stressors, and genetic factors. Cumulative risk assessment methods and approaches are evolving to meet regulatory needs, (MacDonell et al., 2013; Backhaus and Faust 2012; IPCS Workshop 2009) but significant challenges remain. As our understanding of complex exposures and interactions continues to grow, synthesis and integration across disciplines and studies focused on different aspects of the environmental fate–exposure–toxicology–health outcome continuum are required to assess the likelihood of adverse effects and to support cumulative risk assessment. Environmental exposures are virtually always to complex mixtures (Katherine von Stackelberg et al., 2015).
AOP Development Strategy
Context
This AOP was developed as part of an Environmental Protection Agency effort to increase the impact of AOPs published in the peer-reviewed literature, but heretofore unrepresented in the AOP-Wiki, by facilitating their entry and update. The originating work for this AOP was Katherine von Stackelberg & Elizabeth Guzy & Tian Chu & Birgit Claus Henn, 2015. Exposure to Mixtures of Metals and Neurodevelopmental Outcomes: A Multidisciplinary Review Using an Adverse Outcome Pathway Framework, Risk Analysis, John Wiley & Sons, vol. 35(6), pages 971-1016, June. This publication, and the work cited within, were used create and support this AOP and its respective KE and KER pages.
An examination of neurodevelopmental disorders and subclinical effects using multi-domain global neurodevelopment assessments is warranted as they can have profound population level implications. In the context of neurotoxicity, neurodevelopmental pathways in the developing human brain are not fully understood (Schubert et al., 2015; Bal-Price et al., 2015) although there are a number of commonly observed phenomena which may take part in those pathways e.g. changes in intracellular calcium, ROS generation, apoptosis, and neurotransmitter disruption. This AOP highlights a specific set of response-response relationships using a subset of those commonly observed phenonema related to metals and metal mixture exposures leading to deficits in learning and cognition.
The focus of the originating work was to conduct a review of the literature on relationships between prenatal and early life exposure to mixtures of lead (Pb), arsenic (As), cadmium (Cd), and manganese (Mn) with neurodevelopmental outcomes and then use an AOP framework to integrate lines of evidence from multiple disciplines based on evolving guidance developed by the Organization for Economic Cooperation and Development (OECD). Importantly, the review considered whether exposures to mixtures of metals was associated with neurodevelopment effects that were greater or less than effects from exposure to each individual metal.
Strategy
The originating authors conducted a literature search to develop a database of publications categorized by discipline or field of study: toxicology, epidemiology, exposure, and gene-environment interaction. The literature search relied on standard search engines such as PubMed, Web of Science, Google Scholar, Environmental Index, Scopus, Toxline, and Toxnet and the search strategy included terms related to metal mixtures, individual metals (e.g., arsenic, lead, manganese, and cadmium), neurodevelopmental health outcomes, and associated Medical Subject Headings (MeSH) terms. The originating authors reviewed references from individual citations to identify additional studies not captured through the literature search itself. They then included all relevant publications through September 2013. Only studies focused primarily on developmental or neurotoxic endpoints were included; those focused on carcinogenesis or other systemic effects were not included unless there was a particular relevance to a neurotoxic or developmental outcome.
The scope of the aforementioned EPA project was limited to re-representing the AOP(s) as presented in the originating publication. The literature used to support this AOP and its constituent pages began with the originating publication and followed to the primary, secondary, and tertiary works cited therein.

KE and KER page creation and re-use was determined using Handbook principles where page re-use was preferred. Once a baseline level of information was populated for the AOP the authors of the originating publication were contacted for collaboration.
Efforts were made not to editorialize or otherwise add any content to the AOP or its constituent pages that weren’t provided in the primary, secondary, or tertiary literature. In some cases, however, descriptive content was added to pages e.g., assays on a KE page, even if they weren’t specifically provided in the literature stemming from the originating publication.
Summary of the AOP
Events:
Molecular Initiating Events (MIE)
Key Events (KE)
Adverse Outcomes (AO)
| Type | Event ID | Title | Short name |
|---|
| MIE | 2146 | Activation of mitogen-activated protein kinase kinase, extracellular signal-regulated kinase 1/2 | Activation of MEK, ERK1/2 |
| KE | 1339 | Increase, intracellular calcium | Increase, intracellular calcium |
| KE | 2151 | Disruption, neurotransmitter release | Disruption, neurotransmitter release |
| AO | 341 | Impairment, Learning and memory | Impairment, Learning and memory |
Relationships Between Two Key Events (Including MIEs and AOs)
| Title | Adjacency | Evidence | Quantitative Understanding |
|---|
| Activation of MEK, ERK1/2 leads to Increase, intracellular calcium | adjacent | Moderate | |
| Increase, intracellular calcium leads to Disruption, neurotransmitter release | adjacent | Moderate | |
| Disruption, neurotransmitter release leads to Impairment, Learning and memory | adjacent | Moderate |
Network View
Prototypical Stressors
Life Stage Applicability
| Life stage | Evidence |
|---|---|
| All life stages | High |
Taxonomic Applicability
Sex Applicability
| Sex | Evidence |
|---|---|
| Unspecific | Moderate |
Overall Assessment of the AOP
|
1. Support for Biological Plausibility of KERs |
Defining Question |
High (Strong) |
Moderate |
Low (Weak) |
|
|
Is there a mechanistic relationship between KEup and KEdown consistent with established biological knowledge? |
Extensive understanding of the KER based on extensive previous documentation and broad acceptance. |
KER is plausible based on analogy to accepted biological relationships, but scientific understanding is incomplete |
Empirical support for association between KEs , but the structural or functional relationship between them is not understood. |
|
Relationship 2942: Activation of MEK, ERK1/2 (2146) leads to Increase, intracellular calcium (1339) |
Moderate Empirical evidence indicates a complex relationship between MEK, ERK1/2 activation and inhibition and Ca2+ response including Ca2+ feeding back into a ERK1/2 activation. This relationship appears to vary across species and cell type. |
|||
|
Relationship 2954: Increase, intracellular calcium (1339) leads to Disruption, neurotransmitter release (2151) |
Strong Intracellular calcium regulation is broadly known as being an important aspect of a number of processes in a variety of cells and is particularly critical in nerve cell terminals where it mediates transmitter release. |
|||
|
Relationship 2955: Disruption, neurotransmitter release (2151) leads to Impairment, Learning and memory (341) |
Strong The role of various neurotransmitters and receptors in cognitive function and memory formation are well studied.
|
|||
Domain of Applicability
Life Stage
Life stages applicable to this AOP encompass the full life cycle. Many of the key events are measured in pregnant females with the adverse outcome (impairment, learning and memory) measured at all life stages.
Taxonomic Applicability
Most evidence for this AOP is derived from rodents and humans where rodents were selected with their ability to model human responses.
Sex Applicability
This AOP is applicable to all sexes.
Essentiality of the Key Events
|
2. Essentiality of KEs |
Defining question |
High (Strong) |
Moderate |
Low (Weak) |
|
Are downstream KEs and/or the AO prevented if an upstream KE is blocked? |
Direct evidence from specifically designed experimental studies illustrating essentiality for at least one of the important KEs |
Indirect evidence that sufficient modification of an expected modulating factor attenuates or augments a KE |
No or contradictory experimental evidence of the essentiality of any of the KEs. |
|
|
MIE 2146:Activation of MEK, ERK1/2 |
Moderate MEK, ERK1/2 activation is fundamental in delivering signals which regulate the cell cycle, proliferation, differentiation, adhesion, and more. Disruptions in this activation have wide reaching effects however, there is evidence that downstream KEs can also activate this KE. |
|||
|
KE 1339: Increase, intracellular calcium |
High Calcium, as a primary intracellular messenger in neurons and regulator of cell responses to stress has been shown to directly affect neurotransmitter release with manipulation. |
|||
|
KE 2151: Disruption, neurotransmitter release |
High Neurotransmitter receptor blocking experiments have shown to directly impair learning and memory tasks in rodents. |
|||
|
AO 341: Impairment, Learning and memory |
N/A |
|||
|
AOP 499 |
High/Moderate There is direct evidence contained KER 2955. |
|||
Evidence Assessment
|
3. Empirical Support for KERs |
Defining Questions |
High (Strong) |
Moderate |
Low (Weak) |
|
|
Does empirical evidence support that a change in KEup leads to an appropriate change in KEdown? Does KEup occur at lower doses and earlier time points than KE down and is the incidence of KEup > than that for KEdown? Inconsistencies? |
if there is dependent change in both events following exposure to a wide range of specific stressors (extensive evidence for temporal, dose- response and incidence concordance) and no or few data gaps or conflicting data |
if there is demonstrated dependent change in both events following exposure to a small number of specific stressors and some evidence inconsistent with the expected pattern that can be explained by factors such as experimental design, technical considerations, differences among laboratories, etc. |
if there are limited or no studies reporting dependent change in both events following exposure to a specific stressor (i.e., endpoints never measured in the same study or not at all), and/or lacking evidence of temporal or dose- response concordance, or identification of significant inconsistencies in empirical support across taxa and species that don’t align with the expected pattern for the hypothesized AOP |
|
Relationship 2942: Activation of MEK, ERK1/2 (2146) leads to Increase, intracellular calcium (1339) |
Moderate The evidence collection strategy for this AOP focused mainly on metal and metal mixture exposures, of which, there were many that showed dependent change in both these events following exposure. |
|||
|
Relationship 2954: Increase, intracellular calcium (1339) leads to Disruption, neurotransmitter release (2151) |
Moderate The evidence collection strategy for this AOP focused mainly on metal and metal mixture exposures, of which, there were many that showed dependent change in both these events following exposure. |
|||
|
Relationship 2955: Disruption, neurotransmitter release (2151) leads to Impairment, Learning and memory (341) |
Moderate The evidence collection strategy for this AOP focused mainly on metal and metal mixture exposures, of which, there were many that showed dependent change in both these events following exposure. |
|||
Known Modulating Factors
| Modulating Factor (MF) | Influence or Outcome | KER(s) involved |
|---|---|---|
Quantitative Understanding
Considerations for Potential Applications of the AOP (optional)
Developmental neurotoxicity (DNT) is an adverse outcome of concern to multiple regulatory agencies. In vitro screening assays for MEK-ERK1/2 activation would not be recommended as a direct alternative or replacement to established DNT assays like OECD Test No. 426 (OECD 2007). However, detection of MEK-ERK1/2 activation in neuronal cell types may be used to prioritize chemicals with potential to elicit neurotoxicity and flag them for testing in ortogonal assays for evaluating DNT, including proposed alternative test methods (Bal-Price et al. 2018; Crofton et al 2022).
References
Antonio, M. Teresa, Noelia López, and M. Luisa Leret. "Pb and Cd poisoning during development alters cerebellar and striatal function in rats." Toxicology 176.1-2 (2002): 59-66.
Asit Rai and others, Characterization of Developmental Neurotoxicity of As, Cd, and Pb Mixture: Synergistic Action of Metal Mixture in Glial and Neuronal Functions, Toxicological Sciences, Volume 118, Issue 2, December 2010, Pages 586–601, https://doi.org/10.1093/toxsci/kfq266
Backhaus T, Faust M. Predictive environmental risk assessment of chemical mixtures: A conceptual framework. Environmental Science & Technology, 2012; 46(5):2564–2573.
Bal-Price A, Crofton KM, Sachana M, Shafer TJ, Behl M, Forsby A, Hargreaves A, Landesmann B, Lein PJ, Louisse J, Monnet-Tschudi F, Paini A, Rolaki A, Schrattenholz A, Sunol C, van Thriel C, Whelan M, Fritsche E. Putative adverse outcome pathways relevant to neurotoxicity. Critical Reviews in Toxicology, 2015; 45(1):83–91.
Bal-Price A, Hogberg HT, Crofton KM, Daneshian M, FitzGerald RE, Fritsche E, Heinonen T, Hougaard Bennekou S, Klima S, Piersma AH, Sachana M, Shafer TJ, Terron A, Monnet-Tschudi F, Viviani B, Waldmann T, Westerink RHS, Wilks MF, Witters H, Zurich MG, Leist M. Recommendation on test readiness criteria for new approach methods in toxicology: Exemplified for developmental neurotoxicity. ALTEX. 2018;35(3):306-352. doi: 10.14573/altex.1712081. Erratum in: ALTEX. 2019;36(3):506.
Crofton KM, Bassan A, Behl M, Chushak YG, Fritsche E, Gearhart JM, Marty MS, Mumtaz M, Pavan M, Ruiz P, Sachana M, Selvam R, Shafer TJ, Stavitskaya L, Szabo DT, Szabo ST, Tice RR, Wilson D, Woolley D, Myatt GJ. Current status and future directions for a neurotoxicity hazard assessment framework that integrates in silico approaches. Comput Toxicol. 2022 May;22:100223. doi: 10.1016/j.comtox.2022.100223.
International Programme on Chemical Safety (IPCS),World Health Organization (WHO). Assessment of combined exposures to multiple chemicals. Report of a WHO/IPCS International Workshop, 2009.
Izquierdo, Ivan. Role of NMDA receptors in memory. Trends in Pharmacological Sciences 12.4 (1991): 128-129
Katherine von Stackelberg & Elizabeth Guzy & Tian Chu & Birgit Claus Henn, 2015. "Exposure to Mixtures of Metals and Neurodevelopmental Outcomes: A Multidisciplinary Review Using an Adverse Outcome Pathway Framework," Risk Analysis, John Wiley & Sons, vol. 35(6), pages 971-1016, June.
Lupușoru CE, Popa EG, Sandu RB, Buca BR, Mititelu-Tarțău L, Lupușoru RV, The influence of Bidens tripartita extracts on psychomotor abilities and cognitive functions in rats. Farmacia, 2017; 65(2): 284-288.
MacDonell MM, Haroun LA, Teuschler LK, Rice GE, Hertzberg RC, Butler JP, Chang Y-S, Clark SL, John AP, Perry CS, Garcia SS, Jacob JH, Scofield MA. 2013. Cumulative risk assessment toolbox:Methods and approaches for the practitioner. Journal of Toxicology, 2013; Article ID 310904, doi:10.1155/2013/310904.
Navarette M, Perea G, Maglio L, Pastor J, de Sola RG, Araque A. Astrocyte calcium signal and gliotransmission in human brain tissue. Cerebral Cortex, 2013; 23:1240–1246.
Neal, A.P., Guilarte, T.R. Molecular Neurobiology of Lead (Pb2+): Effects on Synaptic Function. Mol Neurobiol 42, 151–160 (2010). https://doi.org/10.1007/s12035-010-8146-0
OECD (2007), Test No. 426: Developmental Neurotoxicity Study, OECD Guidelines for the Testing of Chemicals, Section 4, OECD Publishing, Paris, https://doi.org/10.1787/9789264067394-en.
Schubert D, Martens GJM, Kolk SM. Molecular underpinnings of prefrontal cortex development in rodents provide insights into the etiology of neurodevelopmental disorders. Molecular Psychiatry, 2013; 2014:1–15.