
This AOP is licensed under the BY-SA license. This license allows reusers to distribute, remix, adapt, and build upon the material in any medium or format, so long as attribution is given to the creator. The license allows for commercial use. If you remix, adapt, or build upon the material, you must license the modified material under identical terms.
AOP: 503
Title
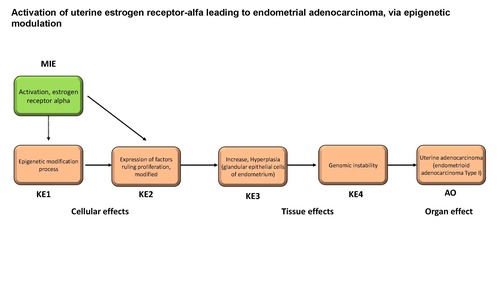
Activation of uterine estrogen receptor-alfa leading to endometrial adenocarcinoma, via epigenetic modulation
Short name
Graphical Representation
Point of Contact
Contributors
- Barbara Viviani
- Miriam Midali
Coaches
OECD Information Table
| OECD Project # | OECD Status | Reviewer's Reports | Journal-format Article | OECD iLibrary Published Version |
|---|---|---|---|---|
| 1.100 | Under Review |
This AOP was last modified on October 06, 2025 09:46
Revision dates for related pages
| Page | Revision Date/Time |
|---|---|
| Activation, estrogen receptor alpha | January 28, 2026 14:32 |
| Epigenetic modification process | April 28, 2025 08:48 |
| Expression of factors ruling proliferation, modified | April 28, 2025 08:51 |
| Increase, Hyperplasia (glandular epithelial cells of endometrium) | September 18, 2025 15:31 |
| Genomic instability | January 03, 2025 11:58 |
| Uterine adenocarcinoma (endometrioid adenocarcinoma Type I) | March 06, 2025 08:39 |
| Activation, ERα leads to Epigenetic modification process | April 28, 2025 08:57 |
| Epigenetic modification process leads to Expression of factors ruling proliferation, modified | March 27, 2025 06:50 |
| Expression of factors ruling proliferation, modified leads to Increase, Hyperplasia (glandular epithelial cells of endometrium) | April 28, 2025 09:01 |
| Increase, Hyperplasia (glandular epithelial cells of endometrium) leads to Genomic instability | March 27, 2025 07:05 |
| Genomic instability leads to endometrioid adenocarcinoma Type I | March 27, 2025 07:12 |
| Tamoxifen | November 29, 2016 18:42 |
| 17beta-Estradiol | November 29, 2016 18:42 |
Abstract
This AOP describes the links between activation of the uterine estrogen receptor alpha and endometrial adenocarcinoma via epigenetic modulation. An evidence-based approach methodology was adopted for this specific purpose. Uterine (endometrial) adenocarcinoma (UA), the most common type of uterine cancer, is a cancer that arises in the layer of cells that make up the inner epithelial lining of the uterus (endometrium). Based on clinical, pathological and molecular characteristics, human uterine adenocarcinomas have been divided into two broad categories: type I and type II. The Molecular Initiating Event (MIE) in this AOP is the activation of the uterine estrogen receptor alpha (ERα). ERα is a receptor covalently bound by estrogens, which upon dimerisation can translocate to the nucleus where it can bind to estrogen-responsive elements and recruit co-activators or co-repressors, which can attract co-regulatory proteins. The mayor KEs are an epigenetic modification process (KE1), the modification of expression of factors ruling proliferation (KE2), than hyperplasia of glandular epithelial cells of endometrium (KE3), than a genomic instability (KE4) than lead to uterine adenocarcinoma (AO).
The outcome is intended to support the identification of substances with endocrine disrupting properties. For this specific purpose, an evidence-based approach methodology was used. The available evidence from the literature was systematically mapped to identify MIEs and KEs associated with AO, independent of prototypical stressors, by means of:
1. a priori defined search strategies initially addressing the AO and biologically plausible MIEs,
2. application of machine learning technique (Topic modelling) that automatically analyzes text data to identify biologically plausible KERs,
3. systematic literature review and critical appraisal of prioritized evidence, taking into account human, in vivo and in vitro studies.
Estradiol and tamoxifen, two recognised human risk factors for endometrioid adenocarcinoma, were used as tool chemicals to empirically support the response and temporal concordance of the identified Key Event Relationships (KERs). All evidence was then integrated using the AOP conceptual network.
AOP Development Strategy
Context
Uterine (endometrial) adenocarcinoma (UA), the most common uterine cancer, is a type of cancer that arises in the layer of cells that form the inner epithelial lining of the uterus (endometrium). Based on clinical, pathological and molecular features, uterine adenocarcinomas in humans have been classified into two broad categories: type I and type II (Sherman, 2000).
Type I adenocarcinoma is the most common type, accounting for approximately 80% of cases. It is often referred as endometrioid adenocarcinoma since it is a well differentiated (low grade) tumor characterized by a glandular growth pattern resembling normal endometrial epithelium. Clinically, is often characterized by a favorable prognosis. Type I adenocarcinoma is estrogen-dependent, estrogen-receptor-positive and arises in a background of endometrial hyperplasia. It can be polypoid or infiltrative, the latter can spread transmurally through the uterine wall and to adjacent organs. Involvement of regional lymph nodes can occur and, in advanced stages, the tumor may metastasize to distant organs including lungs, liver, bones, and other organs (Robbins and Cotran, 2015).
Type II adenocarcinoma comprises about 10-20% of UA cases and has a non-endometrioid morphology. It is non-estrogen-dependent, estrogen-receptor-negative and has a serous, papillary, or clear cell morphology (Sherman, 2000). Clinically, it is characterized by an aggressive clinical course, and a propensity for early spread and poor prognosis (Bansal et al. 2009). It arises in a background of endometrial atrophy. This classification that sub-divide uterine adenocarcinomas into types I and II is not completely accurate since a minority of endometrial cancers may exhibit shared characteristics (Bansal et al. 2009).
The dependence of Type I uterine adenocarcinoma on estrogens is supported by different epidemiologic observations:
- the raised incidence rate for uterine adenocarcinoma in the population in conjunction with widespread use of unopposed estrogen replacement therapy in menopause (Sherman 2000)
- the decreased incidence with the decline in the use of this hormone preparations (Sherman 2000)
- the increased risk of UA as a consequence of the use of oral contraceptives based on estrogens (Weiss et al. 1976; 1980)
- the 2 to 3 times increase in the relative risk of developing UA in patients under tamoxifen therapy, due to its agonistic action on estrogen receptors in endometrial tissue (Passarello et al. 2019).
Prolonged estrogenic stimulation of the endometrium is associated with atypical endometrial hyperplasia (Sherman 2000). Endometrial hyperplasia is characterized by an increased (pathological) proliferation of the endometrial epithelial cells compared with normal proliferative endometrium.
Strategy
Summary of the AOP
Events:
Molecular Initiating Events (MIE)
Key Events (KE)
Adverse Outcomes (AO)
| Type | Event ID | Title | Short name |
|---|
| MIE | 1065 | Activation, estrogen receptor alpha | Activation, ERα |
| KE | 2152 | Epigenetic modification process | Epigenetic modification process |
| KE | 2153 | Expression of factors ruling proliferation, modified | Expression of factors ruling proliferation, modified |
| KE | 772 | Increase, Hyperplasia (glandular epithelial cells of endometrium) | Increase, Hyperplasia (glandular epithelial cells of endometrium) |
| KE | 1896 | Genomic instability | Genomic instability |
| AO | 2154 | Uterine adenocarcinoma (endometrioid adenocarcinoma Type I) | endometrioid adenocarcinoma Type I |
Relationships Between Two Key Events (Including MIEs and AOs)
| Title | Adjacency | Evidence | Quantitative Understanding |
|---|
Network View
Prototypical Stressors
Life Stage Applicability
| Life stage | Evidence |
|---|---|
| Adult |
Taxonomic Applicability
Sex Applicability
| Sex | Evidence |
|---|---|
| Female |
Overall Assessment of the AOP
Domain of Applicability
Essentiality of the Key Events
Evidence Assessment
Known Modulating Factors
| Modulating Factor (MF) | Influence or Outcome | KER(s) involved |
|---|---|---|
Quantitative Understanding
Considerations for Potential Applications of the AOP (optional)
References
Sherman ME (2000). Theories of endometrial carcinogenesis: a multidisciplinary approach. Mod Pathol., 13, 295–308
Robbins and Cotran (2015) Pathologic basis of disease. 9 th Edition.
Bansal N, Yendluri V, and Wenham RM, 2009. The Molecular Biology of Endometrial Cancers and the Implications for Pathogenesis, Classification, and Targeted Therapies. Cancer Control, Vol. 16, No. 1
Weiss NS, Sayvetz TA. Incidence of endometrial cancer in relation to the use of oral contraceptives. N Engl J Med. 1980 Mar 6;302(10):551-4. doi: 10.1056/NEJM198003063021004
Passarello K, Kurian S.,Villanueva V. (2019) Endometrial cancer: an overview of pathophysiology, management and care. Sem. Oncol. Nurs., 35, 157-165
Weiss,N.S., Szekely,D.R. and Austin,D.F. (1976) Increasing incidence of endometrial cancer in the United States. N. Engl. J. Med., 294, 1259–1262