
This AOP is licensed under the BY-SA license. This license allows reusers to distribute, remix, adapt, and build upon the material in any medium or format, so long as attribution is given to the creator. The license allows for commercial use. If you remix, adapt, or build upon the material, you must license the modified material under identical terms.
AOP: 613
Title
Peroxisome proliferator-activated receptor alpha activation leading to early life stage mortality via increased reactive oxygen species production
Short name
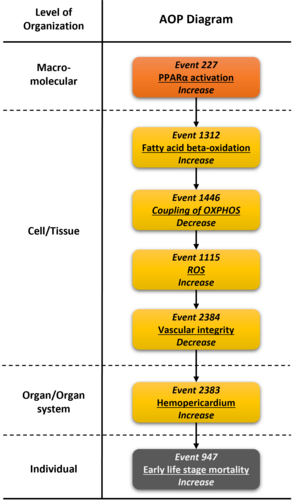
Graphical Representation
Point of Contact
Contributors
- You Song
Coaches
OECD Information Table
| OECD Project # | OECD Status | Reviewer's Reports | Journal-format Article | OECD iLibrary Published Version |
|---|---|---|---|---|
This AOP was last modified on November 07, 2025 05:16
Revision dates for related pages
| Page | Revision Date/Time |
|---|---|
| Activation, PPARα | December 28, 2020 12:48 |
| Increase, Fatty acid beta-oxidation | December 04, 2020 15:21 |
| Decrease, Coupling of oxidative phosphorylation | November 07, 2025 05:15 |
| Increase, Reactive oxygen species | June 12, 2025 01:27 |
| Decrease, Vascular integrity | November 07, 2025 05:42 |
| Increase, Hemopericardium | October 29, 2025 17:05 |
| Increase, Early Life Stage Mortality | March 22, 2018 10:23 |
| Activation, PPARα leads to Increase, Fatty acid β-oxidation | October 30, 2025 04:18 |
| Increase, Fatty acid β-oxidation leads to Decrease, Coupling of OXPHOS | October 30, 2025 04:18 |
| Decrease, Coupling of OXPHOS leads to Increase, ROS | February 25, 2025 11:13 |
| Increase, ROS leads to Decrease, Vascular integrity | October 30, 2025 04:28 |
| Decrease, Vascular integrity leads to Increase, Hemopericardium | October 30, 2025 04:19 |
| Increase, Hemopericardium leads to Increase, Early Life Stage Mortality | October 30, 2025 04:19 |
Abstract
This Adverse Outcome Pathway (AOP) describes the mechanistic sequence linking activation of peroxisome proliferator-activated receptor alpha (PPARα) to early life stage mortality in aquatic vertebrates. Activation of PPARα increases fatty acid β-oxidation, which disrupts oxidative phosphorylation (OXPHOS) coupling and elevates reactive oxygen species (ROS) production. The resulting mitochondrial dysfunction and oxidative stress decrease vascular integrity, induce hemopericardium, and culminate in early developmental mortality. The AOP integrates well-established molecular mechanisms of energy metabolism with observable developmental endpoints in fish embryos. It provides a biologically coherent framework to assess developmental toxicity of PPARα agonists, including per- and polyfluoroalkyl substances (PFAS) and fibrates, supporting new approach methodologies (NAMs), read-across, and next-generation risk assessment applications.
AOP Development Strategy
Context
The AOP was developed to capture the conserved mitochondrial and oxidative stress mechanisms underlying developmental toxicity following PPARα activation. Chemicals such as PFAS, phthalates, and fibrates activate PPARα, enhancing fatty acid oxidation and increasing ROS generation. Disruption of mitochondrial coupling and energy depletion leads to vascular leakage and cardiac defects that contribute to embryo lethality. This AOP builds on existing AOPs describing PPARα activation (Event 227) and oxidative stress (Event 1115) to form a metabolism-centered AOP network relevant to fish early life stages.
Strategy
A systematic evidence-gathering strategy was applied to identify and evaluate studies linking key events (KEs) and key event relationships (KERs):
-
Databases searched: PubMed, AOP-Wiki, OECD AOP-KB, Web of Science (2010–2025).
-
Search terms: PPARα activation, β-oxidation, OXPHOS, ROS, ATP depletion, vascular integrity, hemopericardium, mortality, zebrafish, PFAS, fibrate.
-
Inclusion criteria: Experimental or mechanistic studies demonstrating sequential or causal linkage between KEs, preferably with dose–response or temporal concordance data.
-
Approach: Literature screening and expert evaluation to identify essentiality, biological plausibility, and empirical support consistent with OECD AOP development guidelines (2018).
Summary of the AOP
Events:
Molecular Initiating Events (MIE)
Key Events (KE)
Adverse Outcomes (AO)
| Type | Event ID | Title | Short name |
|---|
| MIE | 227 | Activation, PPARα | Activation, PPARα |
| KE | 1312 | Increase, Fatty acid beta-oxidation | Increase, Fatty acid β-oxidation |
| KE | 1446 | Decrease, Coupling of oxidative phosphorylation | Decrease, Coupling of OXPHOS |
| KE | 1115 | Increase, Reactive oxygen species | Increase, ROS |
| KE | 2384 | Decrease, Vascular integrity | Decrease, Vascular integrity |
| KE | 2383 | Increase, Hemopericardium | Increase, Hemopericardium |
| AO | 947 | Increase, Early Life Stage Mortality | Increase, Early Life Stage Mortality |
Relationships Between Two Key Events (Including MIEs and AOs)
| Title | Adjacency | Evidence | Quantitative Understanding |
|---|
Network View
Prototypical Stressors
Life Stage Applicability
| Life stage | Evidence |
|---|---|
| Embryo | |
| Juvenile |
Taxonomic Applicability
| Term | Scientific Term | Evidence | Link |
|---|---|---|---|
| zebrafish | Danio rerio | NCBI |
Sex Applicability
| Sex | Evidence |
|---|---|
| Unspecific |
Overall Assessment of the AOP
The AOP demonstrates strong biological plausibility, particularly for the upstream molecular and cellular events. Empirical support is robust for PPARα activation, β-oxidation increase, and ROS formation, which are consistently observed across multiple vertebrate species and chemical stressors. Empirical evidence linking oxidative stress to vascular and cardiac outcomes is moderate but reproducible. Quantitative understanding of the relationships between intermediate and apical events is developing, with growing omics and imaging datasets supporting semi-quantitative modeling. The AOP has moderate to high overall confidence and is suitable for screening-level hazard identification and mechanistic read-across.
Domain of Applicability
| Aspect | Applicability |
|---|---|
| Taxa | Teleost fish (e.g., Danio rerio, Oryzias latipes); mechanistic conservation across vertebrates. |
| Life stage | Embryonic and early larval development. |
| Sex | Non-sex-specific (early life stage). |
| Biological systems | Liver (metabolic activation), mitochondria (energy homeostasis), and cardiovascular system (vascular integrity). |
Essentiality of the Key Events
| Key Event | Essentiality Evidence | Type of Evidence |
|---|---|---|
| Event 227: PPARα activation (MIE) | Knockdown or knockout of ppara in zebrafish blocks β-oxidation induction and oxidative stress after exposure to PPARα agonists. | Direct |
| Event 1312: Fatty acid β-oxidation (Increase) | Inhibition of β-oxidation (e.g., by etomoxir) reduces ROS formation and prevents cardiac toxicity. | Direct |
| Event 1446: Coupling of OXPHOS (Decrease) | Reduced mitochondrial membrane potential and respiratory coupling precede ROS accumulation and ATP depletion. | Direct |
| Event 1115: ROS (Increase) | ROS scavengers (e.g., N-acetylcysteine) or antioxidants mitigate vascular and cardiac damage, supporting causality. | Direct |
| Event 2384: Vascular integrity (Decrease) | Vascular permeability assays correlate with ROS burden and cardiac edema severity. | Indirect |
| Event 2383: Hemopericardium (Increase) | Reversible upon antioxidant treatment or metabolic rescue. | Indirect |
| Event 947: Early life stage mortality (Increase) | Occurs downstream of cumulative mitochondrial and vascular dysfunction. | Outcome |
Evidence Assessment
Biological Plausibility
High. The mechanistic sequence aligns with established mitochondrial physiology: excessive fatty acid oxidation leads to electron leakage from the respiratory chain, ROS formation, oxidative damage to vascular endothelium, and developmental cardiac failure. The sequence has strong mechanistic coherence across vertebrates.
Empirical Support
Moderate to high. Multiple independent studies in zebrafish embryos and mammalian hepatocytes demonstrate:
-
PPARα activation induces β-oxidation genes (acox1, cpt1a, echs1).
-
OXPHOS uncoupling and ROS increase within 24–48 hours of exposure.
-
ROS elevation precedes vascular collapse and hemopericardium in fish embryos.
-
Temporal and dose concordance between intermediate and apical events is well supported.
Quantitative Understanding
Moderate. Dose–response data exist for several KEs, including ROS induction and mortality. Transcriptomic and metabolomic data enable modeling of upstream relationships, but quantitative linkage between oxidative stress and vascular integrity remains semi-quantitative.
Known Modulating Factors
| Modulating Factor (MF) | Influence or Outcome | KER(s) Involved |
|---|---|---|
| Antioxidant capacity | High antioxidant levels (e.g., glutathione, superoxide dismutase, catalase) can buffer ROS accumulation, mitigating oxidative stress and reducing vascular and cardiac toxicity. | ROS (increase) → Vascular integrity (decrease); ROS (increase) → Hemopericardium (increase) |
| Oxygen availability | Hypoxic or low-oxygen conditions decrease mitochondrial ROS generation, attenuating downstream vascular effects, while hyperoxia enhances ROS formation and vascular damage. | OXPHOS coupling (decrease) → ROS (increase) |
| Nutritional and metabolic status | Lipid-rich diets or high energy reserves enhance fatty acid β-oxidation and PPARα activation, increasing ROS and developmental toxicity risk. | PPARα activation (increase) → Fatty acid β-oxidation (increase); β-oxidation (increase) → OXPHOS coupling (decrease) |
| Temperature | Elevated temperature increases metabolic rate and mitochondrial respiration, enhancing ROS production and sensitivity to mitochondrial uncoupling. | OXPHOS coupling (decrease) → ROS (increase) |
| Chemical lipophilicity | Highly lipophilic compounds bioaccumulate in lipid-rich tissues, leading to stronger PPARα activation and more pronounced metabolic and oxidative effects. | PPARα activation (increase) → β-oxidation (increase) |
| Developmental stage | Early embryonic stages are more susceptible due to immature antioxidant defenses and higher mitochondrial activity. | ROS (increase) → Vascular integrity (decrease); Vascular integrity (decrease) → Hemopericardium (increase) |
| Mitochondrial density and activity | Tissues with high mitochondrial content (e.g., heart, liver) are more vulnerable to OXPHOS disruption and oxidative injury. | OXPHOS coupling (decrease) → ROS (increase); ROS (increase) → Vascular integrity (decrease) |
| Exposure duration and timing | Prolonged or early developmental exposures amplify cumulative ROS burden and downstream damage, while short, late exposures may be reversible. | Across all KERs—temporal accumulation enhances downstream outcomes |
| Chemical co-exposure (e.g., PPARγ or CAR agonists) | May synergistically amplify or modulate PPARα-driven metabolic changes and oxidative stress. | PPARα activation (increase) → downstream metabolic and ROS pathways |
Quantitative Understanding
While no fully parameterized quantitative AOP model exists, several dose–response relationships are established:
-
ROS levels above ~2× baseline associate with mitochondrial uncoupling and decreased ATP.
-
A ≥40% reduction in ATP or mitochondrial potential predicts vascular leakage onset.
-
Hemopericardium incidence correlates with ROS intensity and exposure concentration (e.g., EC50 ~50–100 µM PFOA-equivalents).
Considerations for Potential Applications of the AOP (optional)
-
Supports chemical read-across and prioritization of PPARα agonists for developmental toxicity screening.
-
Provides mechanistic context for non-animal test methods assessing mitochondrial and oxidative stress pathways.
-
Applicable for Adverse Outcome Network integration with hepatic steatosis and mitochondrial dysfunction AOPs.
-
Facilitates NGRA and IATA development focused on energy metabolism perturbation and oxidative stress biomarkers.