
This Event is licensed under the Creative Commons BY-SA license. This license allows reusers to distribute, remix, adapt, and build upon the material in any medium or format, so long as attribution is given to the creator. The license allows for commercial use. If you remix, adapt, or build upon the material, you must license the modified material under identical terms.
Event: 530
Key Event Title
Decreased, GnRH pulsatility/release
Short name
Biological Context
| Level of Biological Organization |
|---|
| Cellular |
Cell term
| Cell term |
|---|
| gonadtroph |
Organ term
| Organ term |
|---|
| hypothalamus |
Key Event Components
| Process | Object | Action |
|---|---|---|
| hormone secretion | Gonadotropin Releasing Hormone | decreased |
Key Event Overview
AOPs Including This Key Event
| AOP Name | Role of event in AOP | Point of Contact | Author Status | OECD Status |
|---|---|---|---|---|
| DBH inhibition | KeyEvent | Undefined (send email) | Under Development: Contributions and Comments Welcome | |
| α-noradrenergic- reduced fecundity | KeyEvent | Undefined (send email) | Under Development: Contributions and Comments Welcome | |
| Decreased GnRH release leading to increased E2 | KeyEvent | Martina Panzarea (send email) | Under development: Not open for comment. Do not cite | |
| Activation, ERα leads to prolonged estrus cycle via decreased kisspeptin | KeyEvent | John Frisch (send email) | Under development: Not open for comment. Do not cite |
Taxonomic Applicability
Life Stages
| Life stage | Evidence |
|---|---|
| All life stages |
Sex Applicability
| Term | Evidence |
|---|---|
| Unspecific |
Key Event Description
Biological state
At puberty, release of GnRH (gonadotropin releasing hormone) by specific brain areas stimulates the pituitary release of luteinising hormone (LH) and follicle stimulating hormone (FSH) that in turn stimulates gonads (ovary and testes) to release sex hormones (androgens, estrogens, progesterone). This is called hypothalamus pituitary gonads (HPG) axis (Fig. 1).
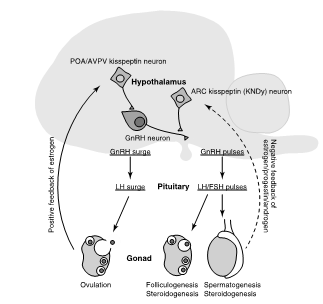
Figure 1. Regulation of reproductive function by the hypothalamus- pituitary-gonadal (HPG) axis. Arrows with solid and broken lines indicate stimulatory and suppressive effects, respectively. ARC, arcuate nucleus; AVPV, anteroventral periventricular nucleus; FSH, follicle-stimulating hormone; GnRH, gonadotropin- releasing hormone; LH, luteinizing hormone; POA, preoptic area (from Matsuda F. et al., 2019)
The master position of GnRH neurons in the hierarchy of signals controlling the gonadotropic axis makes it the final target of a large number of regulators of central (e.g., glutamate, g-amino-butyric acid, neuropeptide Y, noradrenaline) and peripheral (e.g., gonadal steroids, metabolic hormones) origin (Tovar et al., 2006) (Fig. 2).

Figure 2. Mechanisms regulating POA/AVPV kisspeptin neurons, ARC kisspeptin neurons and GnRH neurons. Arrows with solid and broken lines indicate stimulatory and suppressive effects, respectively. ARC, arcuate nucleus; AVP, argi- nine vasopressin; AVPV, anteroventral periventricular nucleus; ERα, estrogen receptor alpha; GABA, γ-aminobutyric acid; GnRH, gonadotropin- releasing hormone; POA, preoptic area; VIP, vasoactive intestinal polypeptide from Matsuda F. et al., 2019. J. Obstet. Gynaecol. Res. Vol. 45, No. 12: 2318–2329, doi:10.1111/JOG.14124.
A key role is played, for example, by estrogens which, however, do not act directly on the GnRH neurons (which do not express E2 receptors), but by modulating the kisspeptin neurons (see also KE:968 in AOP-Wiki).
Kisspeptin ESR1- expressing neurons innervating GnRH neurons are located primarily in the median preoptic nucleus, anteroventral preoptic area (AVPV) and preoptic periventricular nucleus (PeN), 3 contiguous brain regions termed the rostral periventricular area of the third ventricle (RP3V) (see also Fig. 3).

Schematic representation of a parasagittal section of the macaque and human hypothalamus. The arcuate nucleus may be referred to as the infundibular nucleus in the human. The anteroventral nucleus in the preoptic area and the arcuate nucleus in the more caudal medio basal hypothalamus are highlighted in magenta. Redrawn from Yen, 2004 with permission. From Plant, 2012.
Right: Classical model of the hypothalamic control of LH secretion during the ovarian cycle of the rat overlayed on a schematic representation of a parasagittal section of the rat hypothalamus (MBH). The preovulatory LH surge, on the other hand is triggered by a daily circadian signal that originates within the preoptic area, and which is relayed to the GnRH neuronal network only when gated by preovulatory levels of estradiol by the so-called positive feedback action of this steroid. This ensemble of ESR1 neurons innervating the GnRH neuron and their associated glia and afferent inputs is considered here to represent the GnRH surge generator. From Plant, 2012.
The activation of ERα in kisspeptin neurons is an obligatory step in the neural mechanisms mediating release of E2-induced GnRH and LH surges (DuBois et al., 2015). It has been demonstrated that ERα in kisspeptin neurons is required for the positive, but not negative feedback actions of E2 on GnRH/LH secretion in adult female mice (Dubois et al., 2015).
Actually, GnRH neurons do not reside within a discrete brain region but form a dispersed longitudinal array of cells within the medial septum, preoptic area, and hypothalamus. Species differences exist in the caudal limits of this continuum, as most GnRH cells are found in the preoptic area of sheep and rats, but significant numbers exist within the basal hypothalamus of primates. However, retrograde labelling studies have now shown that GnRH neurons throughout this continuum project to the median eminence in rats, sheep, and monkeys (Herbison, 1998).
Biological compartment
GnRH is produced in hypothalamus and released in hypophyseal portal blood system.
General role in biology
The secretion of GnRH triggers sexual maturation, or puberty. GnRH is released in a pulsatile manner into the hypophyseal portal blood system. In the anterior pituitary, GnRH binds to its receptor expressed by gonadotropic cells and induces the release of the two gonadotropins, LH and FSH (Franssen et al., 2021).
There is remarkable consistency across mammals in the pattern of pulsatile secretion with one pulse generated per hour during the follicular/diestrous phase of the cycle and a slower rate of one pulse every 3–4 h following ovulation in the estrous/luteal phase (Herbison, 2018). The only other major change that occurs during the cycle is the occurrence of an abrupt and massive outpouring of GnRH that generates the preovulatory GnRH/LH surge in all spontaneously ovulating mammals (Karsch, et al., 1997, Plant 2012).
Thus, the brain and pituitary produce an on-going pulsatile pattern of gonadotropin secretion that slows on estrous to allow appropriate follicular development and a surge pattern of secretion at mid-cycle to initiate ovulation.
Studies undertaken in rodents suggest that <100 GnRH neurons are sufficient for pulsatile LH secretion and that the most caudally positioned GnRH neuron cell bodies might be preferentially involved in pulse generation (in Herbison, 2016).
Fluctuations in this pattern of GnRH release, combined with alterations in the secretory capacity of the pituitary gonadotrophs, generate the marked changes in LH secretion profile observed over the course of the ovarian cycle (Herbison, 1998; Goodman, 1994).
Low levels of estradiol (E2) inhibit GnRH expression and secretion. In ovariectomized animals, GnRH expression and secretion increase.
In primate (non-human) and mouse both the duration and amplitude of the circulating estradiol signal are critical for the generation of the normal LH surge.
How It Is Measured or Detected
- selective visualization of GnRH neurons with β-galactosidase (Skinner et al., 1999)
- detect living cells tagged with green fluorescent protein (GFP) (Spergel et al., 1999; Kato et al., 2003) or calcium sensors in acute brain-slice preparations (Jasoni et al., 2007).
- sampling of portal blood on a minute-by-minute basis (Evans, 1995).
- microdialysis in rat (Sisk, et al., 2001).
- Immunohistochemistry
- ultra-performance liquid chromatography–tandem mass spectrometry (UPLC–MS/MS)
- in situ hybridization was performed to examine total GnRH mRNA and the primary GnRH heterogeneous nuclear RNA transcript.
- GnRH immunoreactivity and total peptide levels were measured in hypothalamic tissue
- In vitro: commercial RIA kit, sensitivity of the assay was 1 pg/tube.
- Hypothalamic explants
Other
The development of GnRH-secreting neurons from human pluripotent stem cell (hPSC) could be relevant in the future, but these new cells require characterization of their pulsatile secretory properties (Lund et al., 2016 and Lund et al., 2020).
Domain of Applicability
Biological domains of applicability
Endocrine systems with respect to HPG axis, hormone structure, receptors, synthesis pathways, hormonal axes and degradation pathways are well conserved across vertebrate taxa.
Life Stage: Adult, reproductively mature and juveniles.
Sex: Applies to both males and females.
Taxonomic: Primarily studied in laboratory rodents and humans. Plausible for most mammals due to conserved hormone pathways regulating hypothalamus-pituitary-gonadal axis processes. GnRH widespread among vertebrates, including amphibians, reptiles, birds, and mammals (Duan and Allard 2020).
References
Duan C, Allard J. 2020. Gonadotropin-releasing hormone neuron development in vertebrates. General and Comparative Endocrinology. 292: 113465
Dubois SL, Acosta-Martínez M, DeJoseph MR, Wolfe A, Radovick S, Boehm U, Urban JH and Levine JE, 2015. Positive, but not negative feedback actions of estradiol in adult female mice require estrogen receptor α in kisspeptin neurons. Endocrinology, 156:1111-1120. doi: 10.1210/en.2014-1851
Evans NP, McNeilly JR and Webb R, 1995. Effects of indirect selection for pituitary responsiveness to gonadotropin-releasing hormone on the storage and release of luteinizing hormone and follicle-stimulating hormone in prepubertal male lambs. Biol Reprod, 53:237-243. doi: 10.1095/biolreprod53.2.237
Franssen D, Svingen T, Lopez Rodriguez D, Van Duursen M, Boberg J and Parent AS, 2022. A Putative Adverse Outcome Pathway Network for Disrupted Female Pubertal Onset to Improve Testing and Regulation of Endocrine Disrupting Chemicals. Neuroendocrinology, 112:101-114. doi: 10.1159/000515478
Goodman RL (Knobil E NJe), 1994. The neuroendocrine control of the ovine estrous cycle. New York, Raven Press. 659–709 pp.
Herbison AE, 1998. Multimodal influence of estrogen upon gonadotropin-releasing hormone neurons. Endocr Rev, 19:302-330. doi: 10.1210/edrv.19.3.0332
Herbison AE, 2016. Control of puberty onset and fertility by gonadotropin-releasing hormone neurons. Nat Rev Endocrinol, 12:452-466. doi: 10.1038/nrendo.2016.70
Herbison AE, 2018. The Gonadotropin-Releasing Hormone Pulse Generator. Endocrinology, 159:3723-3736. doi: 10.1210/en.2018-00653
Jasoni CL, Todman MG, Strumia MM and Herbison AE, 2007. Cell type-specific expression of a genetically encoded calcium indicator reveals intrinsic calcium oscillations in adult gonadotropin-releasing hormone neurons. The Journal of neuroscience: the official journal of the Society for Neuroscience, 27:860-867. doi: 10.1523/jneurosci.3579-06.2007
Karsch FJ, Bowen JM, Caraty A, Evans NP and Moenter SM, 1997. Gonadotropin-releasing hormone requirements for ovulation. Biol Reprod, 56:303-309. doi: 10.1095/biolreprod56.2.303
Kato M, Ui-Tei K, Watanabe M and Sakuma Y, 2003. Characterization of voltage-gated calcium currents in gonadotropin-releasing hormone neurons tagged with green fluorescent protein in rats. Endocrinology, 144:5118-5125. doi: 10.1210/en.2003-0213
Lund C, Pulli K, Yellapragada V, Giacobini P, Lundin K, Vuoristo S, Tuuri T, Noisa P and Raivio T, 2016. Development of Gonadotropin-Releasing Hormone-Secreting Neurons from Human Pluripotent Stem Cells. Stem Cell Reports, 7:149-157. doi: 10.1016/j.stemcr.2016.06.007
Lund C, Yellapragada V, Vuoristo S, Balboa D, Trova S, Allet C, Eskici N, Pulli K, Giacobini P, Tuuri T and Raivio T, 2020. Characterization of the human GnRH neuron developmental transcriptome using a GNRH1-TdTomato reporter line in human pluripotent stem cells. Disease Models & Mechanisms, 13
Matsuda F, Ohkura S, Magata F, Munetomo A, Chen J, Sato M, Inoue N, Uenoyama Y and Tsukamura H, 2019. Role of kisspeptin neurons as a GnRH surge generator: Comparative aspects in rodents and non-rodent mammals. Journal of Obstetrics and Gynaecology Research, 45:2318-2329. doi: https://doi.org/10.1111/jog.14124
Plant TM, 2012. A comparison of the neuroendocrine mechanisms underlying the initiation of the preovulatory LH surge in the human, Old World monkey and rodent. Front Neuroendocrinol, 33:160-168. doi: 10.1016/j.yfrne.2012.02.002
Sisk CL, Richardson HN, Chappell PE and Levine JE, 2001. In vivo gonadotropin-releasing hormone secretion in female rats during peripubertal development and on proestrus. Endocrinology, 142:2929-2936. doi: 10.1210/endo.142.7.8239
NOTE: Italics indicate edits from John Frisch October 2025. A full list of updates can be found in the Change Log on the View History page.