
This AOP is licensed under the BY-SA license. This license allows reusers to distribute, remix, adapt, and build upon the material in any medium or format, so long as attribution is given to the creator. The license allows for commercial use. If you remix, adapt, or build upon the material, you must license the modified material under identical terms.
AOP: 12
Title
Chronic binding of antagonist to N-methyl-D-aspartate receptors (NMDARs) during brain development leads to neurodegeneration with impairment in learning and memory in aging
Short name
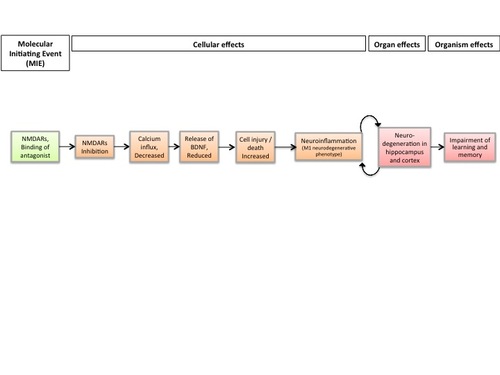
Graphical Representation
Point of Contact
Contributors
- Florianne Tschudi-Monnet
Coaches
OECD Information Table
| OECD Project # | OECD Status | Reviewer's Reports | Journal-format Article | OECD iLibrary Published Version |
|---|---|---|---|---|
| 1.13 | WPHA/WNT Endorsed | Scientific Review | iLibrary link |
This AOP was last modified on April 29, 2023 16:02
Revision dates for related pages
| Page | Revision Date/Time |
|---|---|
| Inhibition, NMDARs | September 16, 2017 10:14 |
| N/A, Neurodegeneration | February 23, 2021 05:07 |
| Decreased, Calcium influx | June 13, 2018 08:26 |
| Binding of antagonist, NMDA receptors | June 13, 2018 08:23 |
| Reduced levels of BDNF | April 04, 2019 09:21 |
| Increase, Cell injury/death | May 27, 2024 07:23 |
| Neuroinflammation | July 15, 2022 09:54 |
| Impairment, Learning and memory | July 26, 2024 09:54 |
| Binding of antagonist, NMDA receptors leads to Inhibition, NMDARs | June 13, 2018 08:48 |
| Inhibition, NMDARs leads to Decreased, Calcium influx | April 07, 2018 04:32 |
| Decreased, Calcium influx leads to BDNF, Reduced | June 13, 2018 09:00 |
| BDNF, Reduced leads to Cell injury/death | November 29, 2016 20:07 |
| Cell injury/death leads to Neuroinflammation | July 15, 2022 08:26 |
| Neuroinflammation leads to N/A, Neurodegeneration | February 23, 2021 05:47 |
| N/A, Neurodegeneration leads to Impairment, Learning and memory | April 11, 2024 15:23 |
| N/A, Neurodegeneration leads to Neuroinflammation | June 13, 2018 09:35 |
| Lead | November 29, 2016 18:42 |
Abstract
This AOP is an extension of AOP 13 linking NMDAR chronic inhibition during brain development to impairment of learning and memory. It links chronic NMDA receptors inhibition during brain development to Adverse Outcomes, i.e. neurodegeneration in hippocampus and cortex with amyloid plaque deposition and tau hyperphosphorylation and impairment of learning and memory, which are considered as hallmark of Alzheimer's disease. It introduces another KE, Neuroinflammation, which is involved in several neurodegenerative diseases. With Neuroinflammation and Neurodegeneration, this AOP connects to AOP 48, where in adult brain, « neuroinflammation » leads to « Neurodegeneration » ; « Neurodegeneration » leads to « Decreased neuronal network function », which finally leads to « Impairement of learning and memory ». Both neurodegeneration and cognitive deficits are observed in Alzheimer’s pathology. But as neurodegenerative diseases are complex and multifactorial, the authors proposed two Adverse outcomes: one at the organism level « Impairment of learning and memory», and one at the organ level, « neurodegeneration ». Both are regulatory endpoints. This AOP integrates in the network of AOPs relative to neurotoxicity testing.
This AOP is based on the hypothesis of Landrigan and coworkers (2005) proposing an early origin of neurodegenerative diseases in later life. The chemical initiator known to block NMDARs and used in this AOP for the empirical support is lead (Pb), which is a well-known developmental neurotoxicant. In epidemiological studies of adults, cumulative lifetime lead exposure has been associated with accelerated decline in cognition (Bakulski et al., 2012), suggesting that long term exposure to lead during brain development or occupational exposure in adulthood increases the risk to develop a neurodegenerative disease of Alzheimer's type. The long latency period between exposure and late-onset of neurodegeneration and cognitive deficits gives a very broad life-stage applicability, where developmental exposure has consequences in the aging brain. Such a long temporal delay between exposure and adverse outcome is a real difficulty and challenge for neurotoxicity testing. As the Key Event « Neuroinflammation » appears to play a crucial role in the neurodegenerative process, the authors propose to include the measurement of this apical KE in the battery of regulation-required neurotoxicity testing.
AOP Development Strategy
Context
Strategy
Summary of the AOP
Events:
Molecular Initiating Events (MIE)
Key Events (KE)
Adverse Outcomes (AO)
| Type | Event ID | Title | Short name |
|---|
| MIE | 201 | Binding of antagonist, NMDA receptors | Binding of antagonist, NMDA receptors |
| KE | 195 | Inhibition, NMDARs | Inhibition, NMDARs |
| KE | 52 | Decreased, Calcium influx | Decreased, Calcium influx |
| KE | 381 | Reduced levels of BDNF | BDNF, Reduced |
| KE | 55 | Increase, Cell injury/death | Cell injury/death |
| KE | 188 | Neuroinflammation | Neuroinflammation |
| AO | 352 | N/A, Neurodegeneration | N/A, Neurodegeneration |
| AO | 341 | Impairment, Learning and memory | Impairment, Learning and memory |
Relationships Between Two Key Events (Including MIEs and AOs)
| Title | Adjacency | Evidence | Quantitative Understanding |
|---|
| Binding of antagonist, NMDA receptors leads to Inhibition, NMDARs | adjacent | High | |
| Inhibition, NMDARs leads to Decreased, Calcium influx | adjacent | Moderate | |
| Decreased, Calcium influx leads to BDNF, Reduced | adjacent | Low | |
| BDNF, Reduced leads to Cell injury/death | adjacent | Low | |
| Cell injury/death leads to Neuroinflammation | adjacent | Moderate | |
| Neuroinflammation leads to N/A, Neurodegeneration | adjacent | Low | |
| N/A, Neurodegeneration leads to Impairment, Learning and memory | adjacent | High | |
| N/A, Neurodegeneration leads to Neuroinflammation | adjacent | Moderate |
Network View
Prototypical Stressors
| Name |
|---|
| Lead |
Life Stage Applicability
| Life stage | Evidence |
|---|---|
| During brain development |
Taxonomic Applicability
Sex Applicability
Overall Assessment of the AOP
The aim of this AOP is to capture the KEs and KERs that occur after chronic binding of antagonist to NMDA receptors in neurons of hippocampus and cortex during brain development and that lead to neurodegeneration with impairment in learning and memory in later life. Neurodegenreation with accumulation of amyloid plaques and hyperphosphorylated tau, as well as cognitive deficit are associated with Alzheimer-type neurodegeneration. Currently, the hypothesis of Landrigan et al., (2005) of developmental origins of neurodegenerative diseases has been demonstrated in monkeys, in rats, mice and in zebrafish following Pb treatment (Zawia and Basha, 2005; Basha and Reddy, 2010; Bihaqi et al., 2014a; Bihaqi et al., 2014b ; Lee and Freeman, 2014). There is strong agreement that Alzheimer's disease is progressive and that neurodegeneration is occuring mainly in hippocampus and cortex, associated with cognitive deficits (Schoemaker et al., 2014). This AOP uses the MIE and several KEs of the AOP 13 entitled "Binding of antagonist to N-methyl-D-aspartate receptors (NMDARs) during brain development induces impairment of learning and memory abilities ", with an additional KE: neuroinflammation and two AOs: an AO at the organ level: Neurodegeneration in hippocampus and cortex and an AO at the organism level: Impairment of learning and memory. Impairment of learning and memory is the same AO as in AOP 13, but the point is that this AO is detected when the brain is aging, and it is due to neurodegeneration with accumulation of amyloid peptides and tau hyperphosphorylation. The recent review by Tartatglione and coworkers (2016) is a very good summary of the challenges and experimental studies described in this AOP.
Developmental Pb exposure has adverse effects on cognitive functioning that can persist into adulthood and may be exacerbated with aging (Schneider et al., 2013). Such delayed effects may be due to epigenetic effects of developmental Pb exposure on DNA methylation mediated at least in part through dysregulation of methyltransferases observed often at the lowest level of exposure (Schneider et al., 2013). In addition, key neurodevelopmental events, such as neural differentiation, cell migration and network formation may be modulated by Pb exposure, predisposing the brain for alterations in higher brain functions, such as learning and memory, and this at different ages (for review, see Aschner et al., 2017). The fact that neuroinflammation triggered during early brain development was shown to cause Alzheimer-like pathology when aging (Krstic et al., 2012), suggests that chronic neuroinflammation may play a causal role in cognitive decline in aging. A recent report described a mechanistic link between chronic inflammation and aging microglia; and a causal role of aging microglia in neurodegenerative cognitive deficits: A sirtuin 1 (SIRT1) deficiency was observed in aging microglia, leading to a selective activation of IL1-b transcription mediated through hypomethylation of IL-1b proximal promoter exacerbating aging or tau-associated cognitive deficits (Cho et al. 2015). Taken together, these data suggest that Pb-induced neuroinflammation during brain development may underlie the delayed effects on cognitive deficits in aging, as depicted in the proposed AOP
Domain of Applicability
This AOP is not sex dependent. Regarding the life stage applicability, MIE induced during brain development can have consequences when brain is aging, according to the hypothesis proposed by Landrigan and coworkers (2005). However, it is also possible that the AO does not depend exclusively on developmental exposure, since cumulative occupational exposure also decreased cognitive functions in aging (Stewart et al., 2006).
Essentiality of the Key Events
Table: Essentiality of KEs
|
2 Support for Essentiality of KEs |
Defining Question Are downstream KEs and/or the AO prevented if an upstream KE is blocked ? |
High (Strong) |
Moderate |
Low (Weak) |
|
Direct evidence from specifically designed experimental studies illustrating essentiality for at least one of the important KEs (e.g. stop/reversibility studies, antagonism, knock out models, etc.) |
Indirect evidence that sufficient modification of an expected modulating factor attenuates or augments a KE leading to increase in KE down or AO |
No or contradictory experimental evidence of the essentiality of any of the KEs |
||
|
KE1 NMDARs inhibition |
STRONG |
Activation of NMDAR results in LTP, which is related to increase synaptic strength and memory formation in hippocampus (Johnston et al., 2009). |
||
|
KE2 Calcium influx decreased |
STRONG |
In CNS, many intracellular responses to modified calcium level are mediated by calcium/calmoduline-regulated protein kinases (Wayman et al., 2008). Mice with a mutation of calmoduline kinase II, which is abundantly found in hippocampus, have shown spatial learning impairment (Silva et al., 1992) |
||
|
KE3 Release of BDNF, reduced |
STRONG |
BDNF serves essential function in synaptic plasticity (Poo, 2001) and is crucial for learning and memory processes (Lu et al., 2008). Precursor form of BDNF and mature BDNF are decreased in the preclinical stages of Alzheimer's disease (Peng et al., 2005) |
||
|
KE4 Cell Injury/death, increased |
STRONG |
Several studies dealing with postnatal administration of NMDAR antagonists such as MK 801, ketamine or ethanol have shown a devastating cell apoptotic degeneration in several brain areas of animal models resulting in learning deficits (Creeley and Olney, 2013) |
||
|
KE5 Neuroinflammation |
MODERATE |
Rationale: Rats treated with Pb from PND 24 to 80 showed a neuroinflammatory response associated with neuronal death in hippocampus and LTP impairment. These effects were significantly reversed by administration of minocycline, an antibiotic known to block microglial reactivity (Liu et al., 2012), demonstrating the essentiality of neuroinflammation for neurodegeneration in hippocampus and impairment of memory processes. In addition, the fact that neuroinflammation triggered during brain development by a systemic immune challenge caused Alzheimer's like pathology (Krstic et al., 2012), showed the central role of neuroinflammation in this pathology. In addition, in a mouse model of Alzheimer's disease, the blockade of microglial cell proliferation and the shifting of the microglial inflammatory profile to an anti-inflammatory phenotype by inhibiting the colony-stimulating factor 1 receptor on microglial cells, prevented synaptic degeneration and improved cognitive functions (Olmos-Alonso et al., 2016). This latter experiment has not been done during brain development. But the hypothesis is that a chronic neuroinflammation during a prolonged period increased the risk to develop an Alzheimer's neurodegenerative disease in aging (Krstic and Knuesel, 2013). However, as other mechanisms such epigenetic modifications can lead to accumulation of amyloid plaques- and tau hyperphosphorylation-related neurodegeneration, and due to some inconsistencies of anti-inflammatory treatments as protection against the neurodegenerative process, the essentiality of Neuroinflammation was considered as moderate. |
||
|
AO (at organ level) Neurodegeneration in hippocampus and cortex |
STRONG |
Several studies descibed Pb-induced accumulation of amyloid peptides and hyperphosphorylated and Pb-induced cell injury/deathin hippocampus or decrease in hippocampal volume, what are all well accepted landmarks of Alzheimer's pathology (Lloret et al., 2015). As described in AOP 48, neurodegeneration can lead to "Decreased neuronal network function" which in turn leads to "impairment of learning and memory", which is also considered as a hallmark of Alzheimer's pathology (Schoemaker et al., 2014). However, there is some controversy about the relationship between increased accumulation of amyloid plaques and increased cognitive deficits: Lichtenstein and coworkers (2010) described that accumulation of amyloid plaques reaches a plateau, whereas a temporal relationship is observed between increased microglial activation, widespread degeneration (decreased hippocampal volume) and increased cognitive deficits. Therefore the essentiality for accumulation of amyloid and tau to cognitive deficits should be considered as moderate. But, as cell injury/death in hippocampus and cortex or decrease in hippocampal volume due to widespread neurodegeneration is strongly associated to impairment in learning and memory, the essentiality of this KE has been rated as strong. |
||
|
AO (at organism level) Impairment of learning and memory |
STRONG |
Neurodegenerative diseases are complex and multifactorial, depend on gene-environment interactions, and have a slow temporal evolution (Sherer et al., 2002; Steece-Collier et al., 2002; Tsang and Soong, 2003); Mutter et al., 2004). A direct association between Pb exposure during brain development and Alzheimer's pathology is not supported by epidemiological studies. However, two studies reported that past adult exposure is linked with neurodegeneration (Stewart et al., 2006) and decline in cognitive function (Schwartz et al., 2000), effects which were observed long after exposure ceases. Tibia lead levels were good predictors of these delayed effects. Another study showed an association between lead exposure early in life with cognitive and behavioral consequences in early adulthood (Agency for toxic substances, 1997). Despite the lack of specific epidemiological evidence, the principle of delayed effects occuring long after exposure, as well as strong evidence from experimental studies (for review, see Chin-Chan et al., 2015) suggest that long-term exposure to environmental toxicants such as Pb during brain development or exposure later in life can be considered as a risk factor for the development of neurodegenerative diseases in aging. |
||
Evidence Assessment
1. Concordance of dose-response and temporal concordance between KEs and the AO
It is difficult to analyze the dose-response relationships between the different KEs, (i) because of the long temporal delay between MIE and AOs ; (ii) because no study has analyzed them simultaneously, and (iii) because of the difficulties in extrapolating in vitro to in vivo data. As the apical KEs and AO occur and can be measured years after exposure, even when Pb blood level has returned to normal, measurement of bone Pb content has been proposed as a measurement of historical Pb exposure in adults (Bakulski et al., 2012, 2014). The following table gives an overview of the doses/concentrations and exposure duration at which the different KEs were measured.
|
KE1 |
KE2 |
KE3 |
KE4 |
K5 |
AO at organ level |
AO at organism level |
|
NMDAR inhibition |
Calcium influx, decreased |
BDNF release, decreased |
Cell injury/death |
Neuroinflammation |
Neurodegeneration with amyloid plaques and tau hyperphosphorylation |
Impairment of learning and memory
|
|
Pb 2.5-5 mM acute inhibits NMDAR whole cell and channel current in hippocampal neurons
(Alkondon et al., 1990) |
Pb 100 nM 1h-24h decrease Ca2+ in embryonic rat hippocampal neurons
(Ferguson et al., 2000) |
No direct evidence |
Pb 2mM in drinking water 3 weeks before mating till weaning (PND 21) resulting in at PND 21 Pb blood 108.8 mg/L Pb hippoc. 0.253 mg/g at PND 91 Pb blood 39.27 mg/L Pb hippoc. 0.196 mg/g
about 35% decrease in synapses in hippocampus
about 30% decrease of hippocampal neurons
(Xiao et al., 2014) |
In vivo 0.22 ppm (together with As and Cd) from gestational day 5 till day 180
in adulthood: IL-1b, TNF-a, IL-6 increased 2x
Ashok et al., 2015
Rats exposed to Pb 100 ppm for 8 weeks (from PND 24 to 80) caused at the end of treatment microglial activation in hippocampus. (Liu et al., 2012
In vitro 10-6-10-4 M for 10 days in 3D cultures of fetal rat brain cells
microglial and astrocyte reactivities
(Zurich et al., 2002)
co-cultures of hippocampal neurons with microglial cells treated with Pb (50 micomol/L for 48h) caused microglial activation and upregulation of IL-1beta, TNF-alpha and i_NOS (Liu et al., 2012) |
Monkeys exposed to Pb 1.5 mg/kg/day from birth to 400 days
at 23 years of age
Tau accumulation Overexpression of amyloid-beta protein precursor and of amyloid-beta enhanced pathologic neurodegeneration
(Bihaqi et al., 2011; Bihaqi and Zawia, 2013)
Mice exposed to Pb 0.2% in drinking water from PND 1-20 or from PND 1-20 + From 3-7 months
at 700 days of age
elevated protein and mRNA for tau and aberrant site-specific tau hyperphosphorylation
(Bihaqi et al., 2014)
Human Tg-SWDI APP transgenic mice , PB 50 mg/kg by gavage for 6 weeks exhibit increase AB in CSF, cortex and hippocampus and increased amyloid plaque load (Gu et al., 2012)
Rats exposed to Pb 100 ppm for 8 weeks (from PND 24 to 80) caused at the end of treatment neuronal death in hippocampus. (Liu et al., 2012)
|
Mice exposed to Pb 0.2% in drinking water from PND 1-20 or from PND 1-20 and from 3-7 months
Tested at 700 days of age
Decrease in cognitive functions (Morris water maze, Y maze testing for spatial memory and memory, a hippocampal formation-dependent task)
(Bihaqi et al., 2014b)
Rats exposed to Pb 100 ppm for 8 weeks (from PND 24 to 80) reduced hippocampal LTP level at the end of the treatment (Liu et al., 2012)
Human Tg-SWDI APP transgenic mice , PB 50 mg/kg by gavage for 6 weeks showed an impaired spatial learning (Gu et al., 2012)
|
2. Strength, consistency and association of AO and MIE
The accepted molecular mechanism of action of the chemical initiator Pb is inhibition of NMDARs (Alkondon et al., 1990; Gavazzo et al., 2001, 2008; Guilarte et al., 1992; Omelchenko et al., 1997) and several experimental studies in rat, monkey and zebrafish linked chronic exposure to Pb during brain development to Alzheimer's-like neurodegeneration with cognitive deficits (Zawia and Basha, 2005; Basha and Reddy, 2010; Bihaqi et al., 2014a; Bihaqi et al., 2014 b; Lee and Freeman, 2014). This AOP is defined by a single environmental chemical, Pb. However, other NMDAR antagonists used as general anesthetics (MK 801, phenylcyclidine, ketamine) applied during brain development may also lead to functional impairments in cognitive domains relevant to memory. The effects of these anesthetics on brain function appear to have a delayed onset, and can be very long-lasting if not permanent. In general, longer durations, higher concentrations and longer or repeated exposures tend to exacerbate impairments (for review, see Walters and Paule, 2017). The mechanisms underlying anesthetic-induced neurotoxicity are unclear, but several hypotheses have been proposed: impairment of mitochondrial integrity and function, dysregulation of intracellular calcium and neuroinflammation have all been implicated (Lei et al., 2012). Some of these mechanisms are common to the KEs described in this AOP, suggesting that such delayed effects on memory processes can be a general consequence of developmental brain exposure to NMDAR inhibitors. However, no studies have yet reported that these other NMDAR inhibitors cause amyloid plaque deposition or tau hyperphosphorylation associated with Alzheimer-like neurodegeneration when aging.
Interestingly, memantine, a NMDAR antagonist used in the treatment of Alzheimer's disease, was shown to improve cognitive functions (for review, see Dekundy, 2006). This might be considered as a discrepancy with the described AOP considering Pb as an antagonist of NMDAR and its potential risk to cause cognitive deficits and amyloid plaque accumulation, which are hallmarks of Alzheimer's disease. However, memantine antagonism of NMDAR is quite different (low affinity and voltage-dependent) and the window of exposure differs completely, since memantine is applied in aged patients when the disease has broken out; whereas the risk of delayed neurodegeneration described in this AOP is due to NMDAR inhibition during brain development.
3. Biological Plausibility, and empirical support
|
|
Defining Question |
High /Strong |
Moderate |
Low/weak |
|
Support for Biological Plausibility of KERs |
Is there a mechanistic (i.e. structural or functional) relationship between KEup and KEdown consistent with established biological knowledge? |
Extensive understanding of the KER based on extensive previous documentation and broad acceptance |
The KER is plausible based on analogy to accept biological relationship but scientific understanding is not completely established |
There is empirical support for a statistical association between KEs but the structural or functional relationship between them is not understood |
|
MIE to KE inhibition of NMDARs |
|
Extensive understanding Limited conflicting data |
|
|
|
KE NMDAR inhibition to KE calcium influx, decreased |
|
Extensive understanding Limitied conflicting data |
|
|
|
KE calcium influx, decreased to KE release of BDNF, decreased |
|
Extensive understanding Limited conflicting data |
|
|
|
KE release of BDNF, decreased to KE Cell Injury/death |
|
Extensive understanding Limited conflicting data |
|
|
|
KE Cell injury/death to KE Neuroinflammation |
|
|
The general mechanisms linking cell injury/death to neuroinflammation is well accepted. However, it is mainly descibed in adult brain. However, a neuroinflammatory response was found following Pb exposure of 3D cultures during synaptogenesis and myelination (Zurich et al., 2002). A controversy exists about apoptosis and neuroinflammation, but some empirical evidences has been provided.
The fact that cell injury/deat leads to neuroinflammation and that neuroinflammation leads to neurodegeneration is known as avicious circle and is involved in neurodegenerative diseases, suggesting that neuroinflammation exacerbates the neurodegenerative process (Griffin et al., 1998; 2006) |
|
|
KE Neuroinflammation to AO Neurodegeneration in Hippocampus and cortex |
In adult, the early involvement of neuroinflammation in the neurodegenerative process is widely accepted.
In immature brain, one study in mice link gestational induction of neuroinflammation to late neurodegeneration with accumulation of aberrant amyloid and tau (Kristic et al., 2012). |
|
There is in vitro experimental data following Pb exposure linking neuroinflammation to extensive neuronal death in immature cells. In vivo, There are several studies linking early Pb exposure to late neurodegeneration in several species. However, the mechanisms involved is epignenetic modifications of genes involved in the amyloid cascade. Such epigenetic modifications may be due to ROS released by the neuroinflammatory process (Bolin et al., 2006). Therefore the link may be indirect and needs further analyses. |
|
|
AO Neurodegeneration in hippocampus and cortex to KE Neuroinflammation |
Concept of vicious circle where neuroinflammation lead to neurodegeneration and vice versa (Griffin et al., 1998, 2006) |
|
|
There are no specific empirical data for the chemical initiator Pb. |
|
AO Neurodegeneration in hippocampus and cortex to AO Impairment of learning and memory |
|
The role of hippocampus in memory processes is well accepted. Alterations of LTP in hippocampus of rats exposed to Pb has been described (Liu et al., 2012), as well as preferential accumulation of hyperphosphorylated tau in frontal cortex of mice exposed during development to Pb. These mice exhibited cognitive deficit when aging (Bihaqi et al., 2014b). |
|
|
Known Modulating Factors
Quantitative Understanding
With an Adverse Outcome occurring after such a long delay after the MIE, it is extremely difficult to make a quantitative link, since the AO can occur when serum Pb levels have returned to normal. Bakulski and coworkers (2012) therefore proposed measuring Pb bone content as an index of historical Pb exposure. Similarly, Schwartz and coworkers (2000) showed that tibia Pb levels were good predictors of delayed cognitive decline of former organolead workers. Thus, Pb blood level is rather representative of acute exposure, whereas Pb bone level represents long-term accumulation (Dorsey et al., 2006).
Regarding the KER "cell injury/death to neuroinflammation", it is accepted that neuronal injury may be sufficient to trigger a neuroinflammatory response. But, because of the neuroprotective or neuroreparative potential of neuroinflammation, it is possible that the consequences of neuroinflammation will be in a first step positive, with microglia expressing the M2 phenotype. After an exposure arrest and a temporal delay (Sandström et al., 2014), or in the presence of cell death (Nakajima and Kohsaka, 2004; Hanish and Kettenmann, 2007), microglia can acquire the M1 neurodegenerative phenotype. Therefore, it is rather the qualitative phenotype of neuroinflammation that will induce neurodegeneration. However, a possible correlation of increased microglial reactivity, measured by PET, and a decrease in hippocampal volume, measured by MRI, suggests, in advanced Alzheimer's disease, a possible link between the intensity of neuroinflammation and the neurodegenerative consequences (Lichtenstein et al., 2010).
Considerations for Potential Applications of the AOP (optional)
This AOP aims at giving a conceptual framework to mechanistically understand an apical hazard, which can occur long after initial exposure; this hazard is not captured in standard regulatory neurotoxicity testing.
The KE "neuroinflammation", which is shared with other AOPs, appears to play an early and central role in the neurodegenerative process (Eikelenboom et al., 2000; Whitton, 2007; Krstic et al., 2012). Neuroinflammation is observed in most neurodegenerative diseases including Alzheimer's disease (Whitton, 2007 ; Tansey and Goldberg, 2009 ; Niranjan, 2014 ; Verkhratsky et al., 2014). Neuroinflammation can also be triggered by several classes of toxicants (Monnet-Tschudi et al., 2007). Any toxicant able to trigger a neuroinflammatory response expressing the neurodegenerative phenotype should be considered as a risk factor for neurodegenerative diseases. Therefore, testing for toxicant-induced neuroinflammation should be used as an endpoint in regulatory toxicology. The standard neurotoxicity testing does not require measurement of any marker of neuroinflammation, except for fuel additives, where testing for a potential increase in glial fibrillary acidic protein (GFAP), as marker of astrocyte reactivity, is mandatory according to US EPA (40 CFR 79 67).
The evolution of regulation towards mechanistically-driven approaches for supporting hazard identification implies also the development of in vitro testing. Three-dimensional cultures, prepared from fetal rat brain cells, exhibiting an histotypic organisation comprising all types of brain cells (specifically microglial cells and astrocytes, as effector cells of neuroinflammation) and allowing long-term maintenance for repeated exposure and for studying the evolution of neuroinflammatory phenotypes, are already available (Alépée et al., 2014; Monnet-Tschudi et al., 2007 ; Sandström et al., 2014). Similar 3D cultures prepared from human pluripotent stem cells are in development (Schwartz et al., 2015; Sandström et al., 2017).
References
ATSDR 2007. Toxicological Profile for Lead. U.S. Agency for Toxic Substances and Disease Registry, August 2007.
Alépée N, Bahinski A, Daneshian M, De Wever B, Fritsche E, Goldberg A, et al. 2014. State-of-the-art of 3D cultures (organs-on-a-chip) in safety testing and pathophysiology. Altex 31(4): 441-477.
Alkondon M, Costa AC, Radhakrishnan V, Aronstam RS, Albuquerque EX. 1990. Selective blockade of NMDA-activated channel currents may be implicated in learning deficits caused by lead. FEBS Lett 261(1): 124-130.
Aschner M, Ceccatelli S, Daneshian M, Fritsche E, Hasiwa N, Hartung T, et al. 2017. Reference compounds for alternative test methods to indicate developmental neurotoxicity (DNT) potential of chemicals: example lists and criteria for their selection and use. Altex 34(1): 49-74.
Ashok A, Rai NK, Tripathi S, Bandyopadhyay S. 2015. Exposure to As-, Cd-, and Pb-mixture induces Abeta, amyloidogenic APP processing and cognitive impairments via oxidative stress-dependent neuroinflammation in young rats. Toxicol Sci 143(1): 64-80.
Bakulski KM, Rozek LS, Dolinoy DC, Paulson HL, Hu H. 2012. Alzheimer's disease and environmental exposure to lead: the epidemiologic evidence and potential role of epigenetics. Curr Alzheimer Res 9(5): 563-573.
Bakulski, K.M., Park, S.K., Weisskopf, M.G., Tucker, K.L., Sparrow, D., Spiro, A., 3rd, Vokonas, P.S., Nie, L.H., Hu, H., Weuve, J., 2014. Lead exposure, B vitamins, and plasma homocysteine in men 55 years of age and older: the VA normative aging study. Environ Health Perspect 122(10), 1066-1074.
Basha R, Reddy GR. 2010. Developmental exposure to lead and late life abnormalities of nervous system. Indian journal of experimental biology 48(7): 636-641.
Bihaqi SW, Huang H, Wu J, Zawia NH. 2011. Infant exposure to lead (Pb) and epigenetic modifications in the aging primate brain: implications for Alzheimer's disease. J Alzheimers Dis 27(4): 819-833.
Bihaqi SW, Zawia NH. 2013. Enhanced taupathy and AD-like pathology in aged primate brains decades after infantile exposure to lead (Pb). Neurotoxicology 39: 95-101.
Bihaqi SW, Bahmani A, Adem A, Zawia NH. 2014a. Infantile postnatal exposure to lead (Pb) enhances tau expression in the cerebral cortex of aged mice: relevance to AD. Neurotoxicology 44: 114-120.
Bihaqi SW, Bahmani A, Subaiea GM, Zawia NH. 2014b. Infantile exposure to lead and late-age cognitive decline: relevance to AD. Alzheimer's & dementia: the journal of the Alzheimer's Association 10(2): 187-195.
Bolin CM, Basha R, Cox D, Zawia NH, Maloney B, Lahiri DK, et al. 2006. Exposure to lead and the developmental origin of oxidative DNA damage in the aging brain. Faseb J 20(6): 788-790.
Chin-Chan, M., Navarro-Yepes, J., Quintanilla-Vega, B., 2015. Environmental pollutants as risk factors for neurodegenerative disorders: Alzheimer and Parkinson diseases. Front Cell Neurosci 9, 124.
Cho SH, Chen JA, Sayed F, Ward ME, Gao F, Nguyen TA, et al. 2015. SIRT1 deficiency in microglia contributes to cognitive decline in aging and neurodegeneration via epigenetic regulation of IL-1beta. J Neurosci 35(2): 807-818.
Creeley CE, Olney JW. (2013) Drug-Induced Apoptosis: Mechanism by which Alcohol and Many Other Drugs can Disrupt Brain Development. Brain Sci. 3: 1153–1181.
Dekundy A.2006. Coadministration of memantine with Acetylcholinesterase Inhibitors: Preclinical and Clinical Evidence. In: Toxicology of Organophosphate and Carbamate Compounds. Gupta RC, editor. Elsevier. Amsterdam. Chap. 4. pp 35-46.
Dorsey CD, Lee BK, Bolla KI, Weaver VM, Lee SS, Lee GS, et al. 2006. Comparison of patella lead with blood lead and tibia lead and their associations with neurobehavioral test scores. Journal of occupational and environmental medicine / American College of Occupational and Environmental Medicine 48(5): 489-496.
Eikelenboom P, Rozemuller AJ, Hoozemans JJ, Veerhuis R, van Gool WA. 2000. Neuroinflammation and Alzheimer disease: clinical and therapeutic implications. Alzheimer Dis Assoc Disord 14 Suppl 1: S54-61.
Ferguson C, Kern M, Audesirk G. 2000. Nanomolar concentrations of inorganic lead increase Ca2+ efflux and decrease intracellular free Ca2+ ion concentrations in cultured rat hippocampal neurons by a calmodulin-dependent mechanism. Neurotoxicology 21(3): 365-378.Gavazzo P, Gazzoli A, Mazzolini M, Marchetti C. 2001. Lead inhibition of NMDA channels in native and recombinant receptors. Neuroreport 12(14): 3121-3125.
Gavazzo P, Zanardi I, Baranowska-Bosiacka I, Marchetti C. 2008. Molecular determinants of Pb2+ interaction with NMDA receptor channels. Neurochem Int 52(1-2): 329-337.
Griffin WST, Sheng JG, Royston MC, Gentleman SM, McKenzie JE, Graham DI, et al. 1998. Glial-neuronal interactions in Alzheimer's disease: The potential role of a 'cytokine cycle' in disease progression. Brain Pathol 8: 65-72.
Griffin WS. 2006. Inflammation and neurodegenerative diseases. Am J Clin Nutr 83(2): 470S-474S.
Gu H, Robison G, Hong L, Barrea R, Wei X, Farlow MR, et al. 2012. Increased beta-amyloid deposition in Tg-SWDI transgenic mouse brain following in vivo lead exposure. Toxicol Lett 213(2): 211-219.
Guilarte TR, Miceli RC. 1992. Age-dependent effects of lead on [3H]MK-801 binding to the NMDA receptor-gated ionophore: in vitro and in vivo studies. Neurosci Lett 148(1-2): 27-30.
Hanisch UK, Kettenmann H. 2007. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci 10(11): 1387-1394.
Johnstone AFM, Gross GW, Weiss D, Schroeder O, Shafer TJ. 20. Use of microelectrode arrays for neurotoxicity testing in the 21st century Neurotoxicology 31: 331-350.
Krstic D, Madhusudan A, Doehner J, Vogel P, Notter T, Imhof C, Manalastas A, Hilfiker M, Pfister S, Schwerdel C, et al. 2012. Systemic immune challenges trigger and drive Alzheimer-like neuropathology in mice. J Neuroinflammation 9:151.
Krstic D, Knuesel I: Deciphering the mechanism underlying late-onset Alzheimer disease. Nat Rev Neurol 2013, 9:25-34.
Landrigan PJ, Sonawane B, Butler RN, Trasande L, Callan R, Droller D. 2005. Early environmental origins of neurodegenerative disease in later life. Environ Health Perspect 113(9): 1230-1233.
Lee J, Freeman JL. 2014. Zebrafish as a model for investigating developmental lead (Pb) neurotoxicity as a risk factor in adult neurodegenerative disease: a mini-review. Neurotoxicology 43: 57-64.
Lei X, Guo Q, Zhang J. 2012. Mechanistic insights into neurotoxicity induced by anesthetics in the developing brain. International journal of molecular sciences 13(6): 6772-6799.
Lichtenstein MP, Carriba P, Masgrau R, Pujol A, Galea E. 2010. Staging anti-inflammatory therapy in Alzheimer's disease. Frontiers in aging neuroscience 2: 142.
Liu, M.C., Liu, X.Q., Wang, W., Shen, X.F., Che, H.L., Guo, Y.Y., Zhao, M.G., Chen, J.Y., Luo, W.J., 2012. Involvement of microglia activation in the lead induced long-term potentiation impairment. PLoS One 7(8), e43924.
Lloret, A., Fuchsberger, T., Giraldo, E., Vina, J., 2015. Molecular mechanisms linking amyloid beta toxicity and Tau hyperphosphorylation in Alzheimers disease. Free Radic Biol Med 83, 186-191.
Monnet-Tschudi F, Zurich MG, Honegger P. 2007. Neurotoxicant-induced inflammatory response in three-dimensional brain cell cultures. Hum Exp Toxicol 26(4): 339-346.
Mutter, J., Naumann, J., Sadaghiani, C., Schneider, R., Walach, H., 2004. Alzheimer disease: mercury as pathogenetic factor and apolipoprotein E as a moderator. Neuro Endocrinol Lett 25(5), 331-339.
Nakajima K, Kohsaka S. 2004. Microglia: Neuroprotective and neurotrophic cells in the central nervous system. Current Drug Targets-Cardiovasc & Haematol Disorders 4: 65-84.
Niranjan R. 2014. The role of inflammatory and oxidative stress mechanisms in the pathogenesis of Parkinson's disease: focus on astrocytes. Mol Neurobiol 49(1): 28-38.
Olmos-Alonso, A., Schetters, S.T., Sri, S., Askew, K., Mancuso, R., Vargas-Caballero, M., Holscher, C., Perry, V.H., Gomez-Nicola, D., 2016. Pharmacological targeting of CSF1R inhibits microglial proliferation and prevents the progression of Alzheimer's-like pathology. Brain 139(Pt 3), 891-907.
Omelchenko IA, Nelson CS, Allen CN. 1997. Lead inhibition of N-methyl-D-aspartate receptors containing NR2A, NR2C and NR2D subunits. J Pharmacol Exp Ther 282(3): 1458-1464.
Peng, S., Wuu, J., Mufson, E.J., Fahnestock, M. 2005. Precursor form of brain-derived neurotrophic factor and mature brain-derived neurotrophic factor are decreased in the pre-clinical stages of Alzheimer's disease. J Neurochem 93(6), 1412-1421.
Poo MM. (2001) Neurotrophins as synaptic modulators. Nat Rev Neurosci. 2: 24–32.
Sandström von Tobel, J., D. Zoia, et al. (2014). "Immediate and delayed effects of subchronic Paraquat exposure during an early differentiation stage in 3D-rat brain cell cultures." Toxicol Lett. DOI : 10.1016/j.toxlet.2014.02.001
Sandstrom J, Eggermann E, Charvet I, Roux A, Toni N, Greggio C, et al. 2017. Development and characterization of a human embryonic stem cell-derived 3D neural tissue model for neurotoxicity testing. Toxicol In Vitro 38: 124-135.
Schneider JS, Kidd SK, Anderson DW. 2013. Influence of developmental lead exposure on expression of DNA methyltransferases and methyl cytosine-binding proteins in hippocampus. Toxicol Lett 217(1): 75-81.
Schoemaker D, Gauthier S, Pruessner JC. 2014. Recollection and familiarity in aging individuals with mild cognitive impairment and Alzheimer's disease: a literature review. Neuropsychology review 24(3): 313-331.
Schwartz BS, Stewart WF, Bolla KI, Simon PD, Bandeen-Roche K, Gordon PB, et al. 2000. Past adult lead exposure is associated with longitudinal decline in cognitive function. Neurology 55(8): 1144-1150.
Schwartz MP, Hou Z, Propson NE, Zhang J, Engstrom CJ, Santos Costa V, et al. 2015. Human pluripotent stem cell-derived neural constructs for predicting neural toxicity. Proc Natl Acad Sci U S A 112(40): 12516-12521.
Sherer, T.B., Betarbet, R., Greenamyre, J.T., 2002. Environment, mitochondria, and Parkinson's disease. Neuroscientist 8(3), 192-197.
Silva AJ, Paylor R, Wehner JM, Tonegawa S. 1992. Impaired spatial learning in alpha-calcium-calmodulin kinase II mutant mice. Science 257: 206-211.
Steece-Collier, K., Maries, E., Kordower, J.H., 2002. Etiology of Parkinson's disease: Genetics and environment revisited. Proc Natl Acad Sci U S A 99(22), 13972-13974.
Stewart WF, Schwartz BS, Davatzikos C, Shen D, Liu D, Wu X, et al. 2006. Past adult lead exposure is linked to neurodegeneration measured by brain MRI. Neurology 66(10): 1476-1484.
Tansey MG, Goldberg MS. 2009. Neuroinflammation in Parkinson's disease: Its role in neuronal death and implications for therapeutic intervention. Neurobiol Dis.
Tartaglione, A.M., Venerosi, A., Calamandrei, G., 2016. Early-Life Toxic Insults and Onset of Sporadic Neurodegenerative Diseases-an Overview of Experimental Studies. Curr Top Behav Neurosci 29, 231-264.
Tsang, F., Soong, T.W., 2003. Interactions between environmental and genetic factors in the pathophysiology of Parkinson's disease. IUBMB Life 55(6), 323-327.
Verkhratsky A, Parpura V, Pekna M, Pekny M, Sofroniew M. 2014. Glia in the pathogenesis of neurodegenerative diseases. Biochemical Society Transactions 42(5): 1291-1301.
Walters JL, Paule MG. 2017. Review of preclinical studies on pediatric general anesthesia-induced developmental neurotoxicity. Neurotoxicol Teratol 60: 2-23.
Wayman GA, Lee YS, Tokomitsu H, Silva A, Soderling TR. (2008) Calmodulin-kinases: modulators of neuronal development and plasticity. Neuron 59: 914-931.
Whitton PS. 2007. Inflammation as a causative factor in the aetiology of Parkinson's disease. Br J Pharmacol 150(8): 963-976.
Xiao Y, Fu H, Han X, Hu X, Gu H, Chen Y, et al. 2014. Role of synaptic structural plasticity in impairments of spatial learning and memory induced by developmental lead exposure in Wistar rats. PLoS One 9(12): e115556.
Zawia NH, Basha MR. 2005. Environmental risk factors and the developmental basis for Alzheimer's disease. Rev Neurosci 16(4): 325
Zurich M-G, Eskes C, Honegger P, Bérode M, Monnet-Tschudi F. 2002. Maturation-dependent neurotoxicity of lead aceate in vitro: Implication of glial reactions. J Neurosc Res 70: 108-116.