
This AOP is licensed under the BY-SA license. This license allows reusers to distribute, remix, adapt, and build upon the material in any medium or format, so long as attribution is given to the creator. The license allows for commercial use. If you remix, adapt, or build upon the material, you must license the modified material under identical terms.
AOP: 281
Title
Acetylcholinesterase Inhibition Leading to Neurodegeneration
Short name
Graphical Representation
Point of Contact
Contributors
- Kendra Conrow
- Karen Watanabe
- Natalia Reyero
- Priscilla Pacheco
- Dennis Sinitsyn
Coaches
OECD Information Table
| OECD Project # | OECD Status | Reviewer's Reports | Journal-format Article | OECD iLibrary Published Version |
|---|---|---|---|---|
This AOP was last modified on September 03, 2023 05:47
Revision dates for related pages
| Page | Revision Date/Time |
|---|---|
| Acetylcholinesterase (AchE) Inhibition | April 29, 2020 17:21 |
| Acetylcholine accumulation in synapses | June 26, 2020 13:06 |
| Increased Muscarinic Acetylcholine Receptors | December 02, 2024 03:35 |
| Overactivation, NMDARs | July 14, 2024 11:45 |
| Increased, Intracellular Calcium overload | June 26, 2020 04:45 |
| Increase, Cell injury/death | May 27, 2024 07:23 |
| Occurrence, Focal Seizure | May 20, 2020 01:40 |
| N/A, Neurodegeneration | February 23, 2021 05:07 |
| Status epilepticus | May 21, 2020 18:26 |
| Increased, glutamate | October 11, 2021 14:58 |
| AchE Inhibition leads to ACh Synaptic Accumulation | September 10, 2023 19:16 |
| ACh Synaptic Accumulation leads to Activation, Muscarinic Acetylcholine Receptors | September 03, 2023 02:35 |
| Activation, Muscarinic Acetylcholine Receptors leads to Occurrence, Focal Seizure | September 03, 2023 02:55 |
| Occurrence, Focal Seizure leads to Increased, glutamate | September 03, 2023 03:04 |
| Increased, glutamate leads to Overactivation, NMDARs | September 03, 2023 03:06 |
| Overactivation, NMDARs leads to Status epilepticus | September 03, 2023 03:19 |
| Status epilepticus leads to Increased, glutamate | September 03, 2023 03:26 |
| Overactivation, NMDARs leads to Increased, Intracellular Calcium overload | September 10, 2023 20:11 |
| Status epilepticus leads to Increased, Intracellular Calcium overload | July 24, 2023 22:52 |
| Increased, Intracellular Calcium overload leads to Cell injury/death | September 03, 2023 05:11 |
| Cell injury/death leads to N/A, Neurodegeneration | September 10, 2023 19:25 |
Abstract
The enzyme acetylcholinesterase (AChE) hydrolyzes acetylcholine (ACh) in order to eliminate it from the body. When AChE is inhibited ACh levels increase. An excess of ACh at cholinergic synapses overstimulates both muscarinic- and nicotinic- receptors (1,2). These receptors are found in most organs in the body, thus the effects of AChE inhibition can result in multiple adverse outcomes affecting a wide variety of functions (1). This AOP focuses upon an acute outcome of neurodegeneration due to AChE inhibition specifically through calcium dysregulation as that has been identified as central to the development of the most severe phenotype caused by acute organophosphate poisoning (3).
1. United States., Environmental Protection Agency., Office of Pesticide Programs. (2000). The Use of Data on Cholinesterase Inhibition for Risk Assessments of Organophosphorous and Carbamate Pesticides. https://www.epa.gov/sites/production/files/2015-07/documents/cholin.pdf accessed Nov. 2018.
2. Quick, M. W., & Lester, R. A. J. (2002). Journal of Neurobiology, 53(4), 457-478. doi:10.1002/neu.10109.
3. Faria et al. (2015). Scientific Reports, 5. doi:10.1038/srep15591.
AOP Development Strategy
Context
Epidemiological studies concerning OP pesticides estimated approximately 3 million cases of acute severe poisoning, as well as 300,000 deaths annually. Most of those deaths occur in developing countries of the Asia-Pacific region (Bertolote et al., 2006). These OP compounds can also be used as chemical warfare nerve agents. The improper use of OP chemicals has tragic consequences such as neurodegeneration, brain damage, and death underscoring the need for safety measures that protect both human health and the environment.
Bertolote, J. M., Fleischmann, A., Eddleston, M. & Gunnell, D. 2006. Deaths from pesticide poisoning: A global response. British Journal of Psychiatry, 189, 201-203. DOI: 10.1192/bjp.bp.105.020834.
Strategy
Construction of AOP 281 started from the bottom up, and involved searching the literature and consultation with experts in neuroscience. Extensive literature searches were conducted in Scopus and Pubmed using keywords such as, “acetylcholinesterase inhibition”, “muscarinic acetylcholine receptor”, “calcium dysregulation”, “organophosphate”, “glutamate”, and “cell death” with an initial focus on zebrafish data. Over 300 papers were reviewed and categorized by whether they contained data to support one or more parts of the AOP. An Excel spreadsheet was used to record reviewed papers and which part(s) of the AOP they supported.
AOP 281 was developed primarily by the authors and experts in this publication, except when existing KEs or KERs are used in the AOP. These existing KEs and KERs have additional authors who are not explicitly cited herein. Three KEs of the 10 in AOP 281 (including the MIE and AO as key events), and eight KERs of eleven were developed specifically for this AOP. The remaining KEs and KERs were modified accordingly to include additional data specific for AOP 281.
Summary of the AOP
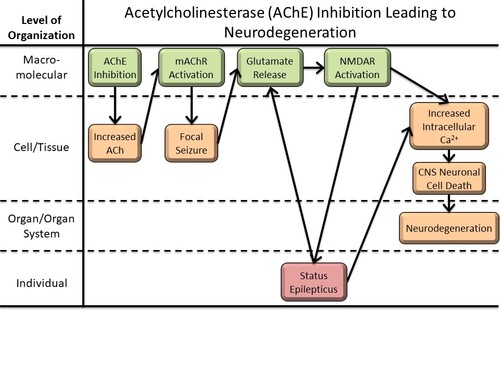
Events:
Molecular Initiating Events (MIE)
Key Events (KE)
Adverse Outcomes (AO)
| Type | Event ID | Title | Short name |
|---|
| MIE | 12 | Acetylcholinesterase (AchE) Inhibition | AchE Inhibition |
| KE | 10 | Acetylcholine accumulation in synapses | ACh Synaptic Accumulation |
| KE | 1602 | Increased Muscarinic Acetylcholine Receptors | Activation, Muscarinic Acetylcholine Receptors |
| KE | 1623 | Occurrence, Focal Seizure | Occurrence, Focal Seizure |
| KE | 1350 | Increased, glutamate | Increased, glutamate |
| KE | 388 | Overactivation, NMDARs | Overactivation, NMDARs |
| KE | 1788 | Status epilepticus | Status epilepticus |
| KE | 389 | Increased, Intracellular Calcium overload | Increased, Intracellular Calcium overload |
| KE | 55 | Increase, Cell injury/death | Cell injury/death |
| AO | 352 | N/A, Neurodegeneration | N/A, Neurodegeneration |
Relationships Between Two Key Events (Including MIEs and AOs)
| Title | Adjacency | Evidence | Quantitative Understanding |
|---|
| AchE Inhibition leads to ACh Synaptic Accumulation | adjacent | High | Moderate |
| ACh Synaptic Accumulation leads to Activation, Muscarinic Acetylcholine Receptors | adjacent | Moderate | High |
| Activation, Muscarinic Acetylcholine Receptors leads to Occurrence, Focal Seizure | adjacent | Moderate | Low |
| Occurrence, Focal Seizure leads to Increased, glutamate | adjacent | Moderate | Low |
| Increased, glutamate leads to Overactivation, NMDARs | adjacent | Moderate | High |
| Overactivation, NMDARs leads to Status epilepticus | adjacent | Moderate | Low |
| Status epilepticus leads to Increased, glutamate | adjacent | Moderate | Low |
| Overactivation, NMDARs leads to Increased, Intracellular Calcium overload | adjacent | High | Moderate |
| Status epilepticus leads to Increased, Intracellular Calcium overload | adjacent | High | Low |
| Increased, Intracellular Calcium overload leads to Cell injury/death | adjacent | High | Low |
| Cell injury/death leads to N/A, Neurodegeneration | adjacent | High | Low |
Network View
Prototypical Stressors
Life Stage Applicability
Taxonomic Applicability
Sex Applicability
Overall Assessment of the AOP
Domain of Applicability
Sex: The AOP is not sex-specific
Life stages: the AOP is relevant to all life stages. Immature or developing populations may be more sensitive due to their increased susceptibility to seizures and developing cholinergic systems.
Taxonomic: given that both cholinergic and glutamatergic systems are highly conserved among vertebrates, this AOP is likely to be applicable to all vertebrates.
Essentiality of the Key Events
All KEs in AOP 281 rank high for essentiality. The provided studies demonstrate direct evidence and include experiments involving inhibition of AChE through the application of various inhibitors, gene-knockout experiments, receptor antagonist studies, and anticonvulsant treatments which are shown to result in the reduction of neurodegeneration.
- AChE Inhibition (MIE) evidence is high. This is supported by several studies that measured increases in ACh after inhibition of AChE by a variety of inhibitors (Del Pino et al., 2017, Karanth et al., 2007, Kim et al., 2003, Kosasa et al., 1999, Ray et al., 2009). Additionally, researchers have demonstrated that pretreatment with a combination of reversible AChE inhibitors, nicotinic and mAChR receptor antagonists prior to exposure to soman resulted in a significantly higher survival rate and overall reduced brain ACh levels compared to controls (Harris et al., 1980).
- ACh accumulation in synapses (KE 1) evidence is high. Blocking the effects of ACh with atropine, an mAChR antagonist, was demonstrated to significantly reduce the pathological effects and neurodegeneration associated with soman intoxication (McDonough et al., 1989).
- Activation of mAChRs (KE 2) evidence is high. M1-mAChR deficient mice through gene-knockout studies were shown to be resistant to seizures induced by pilocarpine, an mAChR agonist (Hamilton et al., 1997).
- Occurrence of Focal Seizure (KE 3) evidence is high. Treatment with diazepam, a GABAA receptor agonist and known anticonvulsant, both prevented seizures and resulted in significantly reduced brain pathology (McDonough et al., 1989).
- Increased Glutamate (KE 4) evidence is high. Application of 500 µM of glutamate showed in reduction in neuron survival, however if NMDA antagonist MK-801 was used in conjunction with glutamate, neuron survival returned to control levels (Michaels and Rothman, 1990). Other in vivo experiments using MK-801 or ketamine demonstrated a reduction in seizure activity and reduced neurodegeneration (Borris et al., 2000, Braitman and Sparenborg, 1989, Sparenborg et al., 1992).
- Overactivation of NMDARs (KE 5) evidence is high. Multiple experiments using ketamine and MK-801, both NMDA receptor antagonists, have been demonstrated to terminate or reduce both seizure activity and neurodegeneration (Borris et al., 2000, Braitman and Sparenborg, 1989, Sparenborg et al., 1992).
- Increased Intracellular Calcium Overload (KE 6) evidence is high. Calcium chelation in zebrafish models of organophosphate exposure significantly reduced neurodegeneration (Faria et al., 2015). Additionally, Deshpande et al. (2008) demonstrated that cell death could be significantly reduced given a low extracellular calcium solution in an in vitro model of SE in rat hippocampal neurons.
- Status Epilepticus (KE 7) evidence follows that of KE 3 and is considered high. Anticonvulsant treatment using diazepam was demonstrated to significantly reduce neurodegeneration (McDonough et al., 1989).
- Cell Injury/Death (KE 8) evidence is considered high. Cell death in the context of the brain is considered a form of neurodegeneration (Przedborski et al., 2003). Therefore, prevention of cell death directly results in the prevention of the adverse outcome.
Evidence Assessment
Biological plausibility: Biological plausibility refers to the structural or functional relationship between the key events based on our fundamental understanding of "normal biology". The evidence for biological plausibility throughout this AOP from inhibition of AChE to neurodegeneration is high. It is well understood that inhibition of AChE is followed by an accumulation of ACh, which subsequently leads to activation of muscarinic acetylcholine receptors and focal seizures. The seizures then lead to increased glutamate, which binds to and overactivates NMDARs. Following that step, we find the highest biological uncertainty in the pathway, with moderate biological plausibility (from overactivation of NMDARs leading to status epilepticus to increased glutamate). The rest of the pathway is considered of high biological plausibility all the way to neurodegeneration.
Concordance of dose-response relationships: Dose response concordance considers the degree to which upstream events are shown to occur at test concentrations equal to or lower than those that cause significant effects on downstream key events, the underlying assumption being that all KEs can be measured with equal precision. There is a significant amount of quantitative data providing dose and temporal concordance for multiple species between AChE inhibitors and the resulting percent AChE inhibition and ACh concentration (Kosasa et al., 1999). Dose-response relationships have been well stablished by showing that AChE inhibition resulted in the progressive accumulation of extracellular ACh. Furthermore, the relationship between increased intracellular calcium and cell death through dose and temporal concordance has also been demonstrated. Additionally, Faria et al. (2015) demonstrated a dose-response relationship between increasing doses of the organophosphate chlorpyrifos-oxon and the prevalence of a severe phenotype marked by measurably increased necrosis.
Temporal concordance: Temporal concordance refers to the degree to which the data support the hypothesized sequence of the key events; i.e., the effect on KE1 is observed before the effect on KE2, which is observed before the effect on KE3 and so on. Temporal concordance has been shown between seizure activity and increasing levels of glutamate (KE4 and KE7). Furthermore, temporal concordance has also been established between status epilepticus and increased intracellular calcium in rats. The relationship between increased intracellular calcium and cell death through dose and temporal concordance has also been demonstrated.
Consistency: We are not aware of cases where the whole chain of key events described was observed without also observing a significant impact on neurodegeneration. Nevertheless, the final adverse outcome is not specific to this AOP. Many of the key events included in this AOP overlap with AOPs linking other molecular initiating events to other adverse outcomes.
Uncertainties, inconsistencies, and data gaps: The current main uncertainties within this AOP are related to seizures and the location of AChE inhibition. Even though it is well known that there are two phases of seizure activity driven by cholinergic and glutamatergic mechanisms, the transition between these phases is not well understood. Additionally, the levels of increasing glutamate post-AChE inhibition appear to be dependent on the location of inhibition as well as stressor specific.
Known Modulating Factors
| Modulating Factor (MF) | Influence or Outcome | KER(s) involved |
|---|---|---|
| butylcholinesterase enzyme | Competes with acetylcholinesterase (ACh) for substrate | 1 |
Quantitative Understanding
At present, the quantitative understanding of this AOP varies by level of biological organization. While the initial KEs leading to activation of muscarinic acetylcholine receptors have a high level of quantitative understanding, following KEs leading all the way to the Adverse Outcome (Cell Injury / Death Leading to Neurodegeneration) have a much lower quantitative understanding. The exception would be KE5 (Increased Glutamate leading to Overactivation of NMDARs), that has multiple kinetic models available to evaluate quantitative relationships. Overall, better quantitative relationships need to be developed to be able to quantitively and effectively predict the adverse outcome.
Considerations for Potential Applications of the AOP (optional)
References
Borris, D. J., Bertram, E. H. & Kapur, J. 2000. Ketamine controls prolonged status epilepticus. Epilepsy Res, 42, 117-22. DOI: 10.1016/s0920-1211(00)00175-3.
Braitman, D. J. & Sparenborg, S. 1989. MK-801 protects against seizures induced by the cholinesterase inhibitor soman. Brain Research Bulletin, 23, 145-148. DOI: 10.1016/0361-9230(89)90173-1.
Del Pino, J., Moyano, P., Díaz, G. G., Anadon, M. J., Diaz, M. J., García, J. M., Lobo, M., Pelayo, A., Sola, E. & Frejo, M. T. 2017. Primary hippocampal neuronal cell death induction after acute and repeated paraquat exposures mediated by AChE variants alteration and cholinergic and glutamatergic transmission disruption. Toxicology, 390, 88-99. DOI: 10.1016/j.tox.2017.09.008.
Deshpande, L. S., Lou, J. K., Mian, A., Blair, R. E., Sombati, S., Attkisson, E. & DeLorenzo, R. J. 2008. Time course and mechanism of hippocampal neuronal death in an in vitro model of status epilepticus: role of NMDA receptor activation and NMDA dependent calcium entry. Eur J Pharmacol, 583, 73-83. DOI: 10.1016/j.ejphar.2008.01.025.
Faria, M., Garcia-Reyero, N., Padrós, F., Babin, P. J., Sebastián, D., Cachot, J., Prats, E., Arick Ii, M., Rial, E., Knoll-Gellida, A., Mathieu, G., Le Bihanic, F., Escalon, B. L., Zorzano, A., Soares, A. M. & Raldúa, D. 2015. Zebrafish Models for Human Acute Organophosphorus Poisoning. Sci Rep, 5, 15591. DOI: 10.1038/srep15591.
Hamilton, S. E., Loose, M. D., Qi, M., Levey, A. I., Hille, B., McKnight, G. S., Idzerda, R. L. & Nathanson, N. M. 1997. Disruption of the m1 receptor gene ablates muscarinic receptor-dependent M current regulation and seizure activity in mice. Proceedings of the National Academy of Sciences, 94, 13311-13316. DOI: 10.1073/pnas.94.24.13311.
Harris, L. W., Stitcher, D. L. & Heyl, W. C. 1980. The effects of pretreatments with carbamates, atropine and mecamylamine on survival and on soman-induced alterations in rat and rabbit brain acetylcholine. Life Sci, 26, 1885-91. DOI: 10.1016/0024-3205(80)90617-7.
Karanth, S., Liu, J., Ray, A. & Pope, C. 2007. Comparative in vivo effects of parathion on striatal acetylcholine accumulation in adult and aged rats. Toxicology, 239, 167-179. DOI: https://doi.org/10.1016/j.tox.2007.07.004.
Kim, Y. K., Koo, B. S., Gong, D. J., Lee, Y. C., Ko, J. H. & Kim, C. H. 2003. Comparative effect of Prunus persica L. BATSCH-water extract and tacrine (9-amino-1,2,3,4-tetrahydroacridine hydrochloride) on concentration of extracellular acetylcholine in the rat hippocampus. J Ethnopharmacol, 87, 149-54. DOI: 10.1016/s0378-8741(03)00106-5.
Kosasa, T., Kuriya, Y., Matsui, K. & Yamanishi, Y. 1999. Effect of donepezil hydrochloride (E2020) on basal concentration of extracellular acetylcholine in the hippocampus of rats. European Journal of Pharmacology, 380, 101-107. DOI: 10.1016/S0014-2999(99)00545-2.
McDonough, J. H., Jr., Jaax, N. K., Crowley, R. A., Mays, M. Z. & Modrow, H. E. 1989. Atropine and/or diazepam therapy protects against soman-induced neural and cardiac pathology. Fundam Appl Toxicol, 13, 256-76. DOI: 10.1016/0272-0590(89)90262-5.
Michaels, R. L. & Rothman, S. M. 1990. Glutamate neurotoxicity in vitro: antagonist pharmacology and intracellular calcium concentrations. J Neurosci, 10, 283-92. DOI: 10.1523/jneurosci.10-01-00283.1990.
Przedborski, S., Vila, M. & Jackson-Lewis, V. 2003. Neurodegeneration: what is it and where are we? J Clin Invest, 111, 3-10. DOI: 10.1172/jci17522.
Ray, A., Liu, J., Karanth, S., Gao, Y., Brimijoin, S. & Pope, C. 2009. Cholinesterase inhibition and acetylcholine accumulation following intracerebral administration of paraoxon in rats. Toxicology and Applied Pharmacology, 236, 341-347. DOI: 10.1016/j.taap.2009.02.022.
Sparenborg, S., Brennecke, L. H., Jaax, N. K. & Braitman, D. J. 1992. Dizocilpine (MK-801) arrests status epilepticus and prevents brain damage induced by soman. Neuropharmacology, 31, 357-68. DOI: 10.1016/0028-3908(92)90068-z.