
This AOP is licensed under the BY-SA license. This license allows reusers to distribute, remix, adapt, and build upon the material in any medium or format, so long as attribution is given to the creator. The license allows for commercial use. If you remix, adapt, or build upon the material, you must license the modified material under identical terms.
AOP: 348
Title
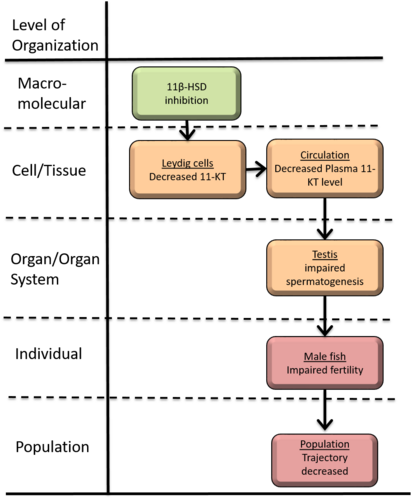
Inhibition of 11β-Hydroxysteroid Dehydrogenase leading to decreased population trajectory
Short name
Graphical Representation
Point of Contact
Contributors
- Young Jun Kim
- Chang-Beom Park
Coaches
- Dan Villeneuve
OECD Information Table
| OECD Project # | OECD Status | Reviewer's Reports | Journal-format Article | OECD iLibrary Published Version |
|---|---|---|---|---|
| 1.93 | Under Development |
This AOP was last modified on April 29, 2023 16:03
Revision dates for related pages
| Page | Revision Date/Time |
|---|---|
| Inhibition of 11β-HSD | July 13, 2020 09:23 |
| Decreased, plasma 11-ketotestosterone level | May 24, 2022 13:51 |
| Impaired, Spermatogenesis | April 10, 2024 17:41 |
| Decrease, Population growth rate | January 03, 2023 09:09 |
| decreased, Fertility | December 17, 2024 15:46 |
| Inhibition of 11β-HSD leads to Decreased, 11KT | July 13, 2020 09:31 |
| Decreased, 11KT leads to Impaired, Spermatogenesis | April 19, 2021 13:32 |
| Impaired, Spermatogenesis leads to decreased, Fertility | August 15, 2022 02:44 |
| decreased, Fertility leads to Decrease, Population growth rate | March 26, 2021 15:24 |
| N-(5-Hydroxytricyclo[3.3.1.13,7]dec-2-yl)-α,α-dimethyl-4-[5-(trifluoromethyl)-2-pyridinyl]-1-piperazineacetamide | July 05, 2020 11:13 |
| Carbenoxolone | March 30, 2020 15:25 |
| Glycyrrhizin | July 13, 2020 10:41 |
| PF915275 | July 13, 2020 10:42 |
Abstract
This AOP links inhibition of 11βHSD to reproductive toxicity in fish. This AOP describes impaired spermatogenesis that may result from the inhibition of 11βHSD. Chemical inhibition of 11βHSD, the molecular-initiating event (MIE), results in decreased 11-KT and cortisone synthesis. The reduction of 11-KT induces the cumulative cortisol by enzymatic conversion insufficiency of cortisone, which leads to decreased spermatogonial proliferation. Impaired fertility is a significant endpoint for evaluation of reproductive toxicity caused by endocrine disruption. It can be used as an endpoint for endocrine disruptor screening. Therefore, this AOP would be useful to identify chemicals with known potential to affect male fish fertility.
AOP Development Strategy
Context
In fish spermatogenesis,11-KT is the main androgen in teleosts, where it has functions in spermatogenesis and their main action of 11-beta dehydrogenase(11βHSD Type 2) is generally regarded as the induction of sperm maturation. it has also its role is to protect these tissues from an excess of cortisol. Stress conditions or inhibition of 11bHSD dehydrogenase activities result in a cortisol excess in the Leydig cells. A surplus of glucocorticoids causes delayed genomic repression of 11KT production through GR or a rapid nongenomic decrease in 11 KT production. The rapid depression has been hypothesized to occur via the putative plasma membrane corticosteroid receptor11βHSD2 is unidirectional with NAD+ as a cofactor. It is expressed not only in mineralocorticoid sensitive tissues such as testis.11βHSD has enzyme activities, metabolizing cortisol to cortisone, and 11 beta -hydroxytestosterone to 11-ketotestosterone (11-KT) which is the main androgen functions spermatogenesis. Especially, spermatogenesis can induce by 11-ketotestosterone(11-KT), a significant androgen in teleost. However, excess circulating cortisol, which is produced by 11β-hydroxylase and decline of 11KT by 11βHSD inhibition, leads to inhibition of the DNA replication in spermatogonial mitosis, gonadal function, and spermatogonial proliferation in male fish.
Acknowledgements: This research was supported by the National Research Council of Science & Technology(NST) grant by the Korea government (MSIP) (No. CAP-17-01-KIST Europe)
Strategy
Summary of the AOP
Events:
Molecular Initiating Events (MIE)
Key Events (KE)
Adverse Outcomes (AO)
| Type | Event ID | Title | Short name |
|---|
| MIE | 1799 | Inhibition of 11β-HSD | Inhibition of 11β-HSD |
| KE | 1756 | Decreased, plasma 11-ketotestosterone level | Decreased, 11KT |
| KE | 1758 | Impaired, Spermatogenesis | Impaired, Spermatogenesis |
| KE | 406 | decreased, Fertility | decreased, Fertility |
| AO | 360 | Decrease, Population growth rate | Decrease, Population growth rate |
Relationships Between Two Key Events (Including MIEs and AOs)
| Title | Adjacency | Evidence | Quantitative Understanding |
|---|
| Inhibition of 11β-HSD leads to Decreased, 11KT | adjacent | High | Moderate |
| Decreased, 11KT leads to Impaired, Spermatogenesis | adjacent | High | Moderate |
| Impaired, Spermatogenesis leads to decreased, Fertility | adjacent | High | High |
| decreased, Fertility leads to Decrease, Population growth rate | adjacent | High | High |
Network View
Prototypical Stressors
Life Stage Applicability
| Life stage | Evidence |
|---|---|
| Adult, reproductively mature | Moderate |
Taxonomic Applicability
| Term | Scientific Term | Evidence | Link |
|---|---|---|---|
| fish | fish | High | NCBI |
Sex Applicability
| Sex | Evidence |
|---|---|
| Male | High |
Overall Assessment of the AOP
11KT has functions in spermatogenesis and their main action of 11βHSD Type 2 is generally regarded as the induction of sperm maturation thus,This AOP will start with reviews about inhibitors of 11βHSD2 as MIE. Stressors for inhibiton were found in table and 11βHSD1 inhibitors were also denoted in previous studies (Jana Vitku et al 2016) . We will further find KERs with Androgen antagonisms and their impact on male fish.
|
To do |
Expected duration |
|
|
Building the AOP frame |
Development of KEs |
6 month |
|
Production of experimental data using inhibitors |
18 month |
|
|
Overall assessment of the AOP |
Biological domain of applicability |
3 month |
|
Essentiality of all KEs |
3 month |
|
|
Evidence supporting all KERs |
5 month |
|
|
Quantitative WoE considerations |
5 month |
|
|
Quantitative understanding for each KER |
6 month |
|
Domain of Applicability
Domain(s) of Applicability
Chemical: This AOP applies to inhibitors of 11KT (Androgen antagonists). Compounds which can bind the 11βHSD as follows
| Inhibitors of 11bHSD2 | Testing system | IC50(μM) |
| (1E,4E)-1,5-Bis(3-methylthiophen-2-yl)penta-1,4-dien-3-one | Human microsomes | 19.58 |
| Abietic acid | HEK 293 cells | 12 |
| Zearalenone | HEK 293 cells | 107 |
| Fusidic acid | HEK 293 cells | 134 |
| Euphane-3b,20-dihydroxy-24-ene | HEK 293 cells | 8.18 |
| Kansuinone | HEK 293 cells | 2.63 |
| Euphol | HEK 293 cells | 0.4 |
| Kansenone | HEK 293 cells | 0.11 |
| (24R)-Eupha-8,25-diene-3b,24-diol | HEK 293 cells | 1.69 |
| (20R,23E)-Eupha-8,23-diene-3b,25-diol | HEK 293 cells | 0.67 |
| Carbenoxolone | CHO cells | 0.02 |
| Endosulfan | HEK 293 cells | 61 |
| BPA | HEK 293 cells | 50 |
| Disulfiram | HEK 293 cells | 0.13 |
| Thiram | HEK 293 cells | 0.13 |
| Diethyldithiocarbamate (DEDTC) | HEK 293 cells | 1.7 |
| HEK 293 cells | 6.3 | |
| Pyrrolidine dithiocarbamate (PDTC) | HEK 293 cells | 6.3 |
| Maneb | HEK 293 cells | 0.75 |
| Zineb | HEK 293 cells | 1.42 |
| Diphenyltin | HEK 293 cells | 2.89 |
| Human microsomes | 3.3 | |
| HEK 293 cells | 3.19 | |
| Triphenyltin | HEK 293 cells | 0.99 |
| Human microsomes | 16.5 | |
| HEK 293 cells | 1.9 | |
| Tributyltin | HEK 293 cells | 1.52 |
| HEK 293 cells | 1.95 | |
| Dibutyltin | HEK 293 cells | 5.03 |
| Human microsomes | 8.9 | |
| 4-t-Octylphenol | HEK 293 cells | 30 |
| Human microsomes | 20.3 | |
| 4-Nonylphenol | HEK 293 cells | 79 |
| 4-n-Octylphenol | Human microsomes | 23.5 |
| 4-n-Nonylphenol | Human microsomes | 26.2 |
| Dicyclohexyl phtalate | Human microsomes | 46.5 |
| Rat microsomes | 32.64 | |
| Dipropyl phthalate | Rat microsomes | 85.59 |
| Di-n-butyl phthlate | Rat microsomes | 13.69 |
| Mono(2-ethylhexyl)phthalate | Rat microsomes | 121.8 |
| Mono(2-ethylhexyl)phthalate | Human microsomes | 110.8 |
| Perfluorooctyl sulphonate | Human microsomes | 0.05 |
| Rat microsomes | 0.29 | |
| Perfluorooctanoic acid | Human microsomes | 24.41 |
| Rat microsomes | 3.8 | |
| Perfluorohexanesulfonate | Human microsomes | 18.97 |
| Rat microsomes | 62.87 | |
| 2-Bis(p-hydroxyphenyl)-1,1,1-trichloroethane | Human microsomes | 55.57 |
| Rat microsomes | 12.96 | |
| Source: Journal of Steroid Biochemistry & Molecular Biology 2016 Jan;155(Pt B):207-16 | ||
Sex: The AOP applies to males only.
Life stages: The relevant life stages for this AOP are reproductively mature spermatogenesis.
Taxonomic: At present, the assumed taxonomic applicability domain of this AOP is iteroparous teleost fish species.
Essentiality of the Key Events
- Many studies showed that the essentiality of the proposed sequence of key events in teleost.
- The essentiality of the proposed negative regulation of 11βHSD is supported by experimental work that evaluated the ability of inhibition to reduce 11KT production in vitro and in vivo
Evidence Assessment
Known Modulating Factors
| Modulating Factor (MF) | Influence or Outcome | KER(s) involved |
|---|---|---|
Quantitative Understanding
Considerations for Potential Applications of the AOP (optional)
A decrease in 11keto levels leads afterward to adverse changes in spermatogenesis. Therefore, it seems that 11βHSD could be a target for EDs and this could be one of the possible mechanisms of endocrine disruption in the testis that leads to impaired spermatogenesis. Consequently, this AOP can be applied to the prediction of VMG-eco and relevant to EDTA caused by the inhibition of 11β-HSD.
References
- Roles of 11β-Hydroxysteroid Dehydrogenase in Fish Spermatogenesis. Endocrinology 147(11):5139–5146
- Hormonal induction of all stages of spermatogenesis in vitro in the male Japanese eel (Anguilla japonica). Proc Natl Acad Sci USA 88:5774–5778
- 17α,20 β Dihydroxy-4-pregnen-3-one: plasma levels during sexual maturation and in vitro production by the testes of amago salmon (Oncorhynchus rhodurus) and rainbow trout (Salmo gairdneri). Gen Comp Endocrinol 51:106–11
- Steroid profiles during spawning in male common carp. Gen Comp Endocrinol 80:223–231
- 11 β-Hydroxysteroid dehydrogenase complementary deoxyribonucleic acid in rainbow trout: cloning, sites of expression, and seasonal changes in gonads. Endocrinology 144:2534–2545
- 11 β –Hydroxysteroid dehydrogenase is a predominant reductase in intact Leydig cells. J Endocrinol 159:233–238
- Large-scale transcriptome sequencing reveals novel expression patterns for key sex-related genes in a sex-changing fish. Biology of Sex Differences 6:26
- Sex Steroids and Their Involvement in the Cortisol-Induced Inhibition of Pubertal Development in Male Common Carp, BIOLOGY OF REPRODUCTION 67, 465–472
- Natural sex change in fish Current Topics in Developmental Biology, Volume 134
- Absence of 11-keto reduction of cortisone and 11-ketotestosterone in the model organism zebrafish Journal of Endocrinology 232,323–335
- Endocrine disruptors and other inhibitors of 11b-hydroxysteroid dehydrogenase 1 and 2: Tissue-specific consequences of enzyme inhibition.Journal of Steroid Biochemistry & Molecular Biology 155(Pt B):207-16.