
This AOP is licensed under the BY-SA license. This license allows reusers to distribute, remix, adapt, and build upon the material in any medium or format, so long as attribution is given to the creator. The license allows for commercial use. If you remix, adapt, or build upon the material, you must license the modified material under identical terms.
AOP: 629
Title
Increased 11β-Hydroxysteroid dehydrogenase type 1 activity leading to MASLD progression via lipogenesis-associated endoplasmic reticulum stress
Short name
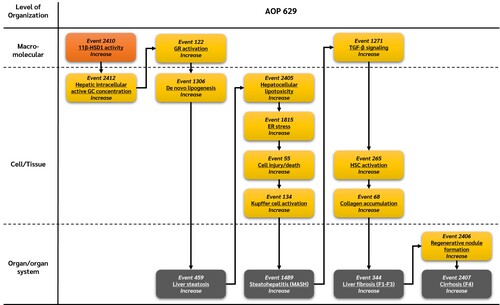
Graphical Representation
Point of Contact
Contributors
- You Song
Coaches
- Shihori Tanabe
OECD Information Table
| OECD Project # | OECD Status | Reviewer's Reports | Journal-format Article | OECD iLibrary Published Version |
|---|---|---|---|---|
| Under Development |
This AOP was last modified on February 26, 2026 04:24
Revision dates for related pages
| Page | Revision Date/Time |
|---|---|
| Increase, 11β-Hydroxysteroid dehydrogenase type 1 activity | February 18, 2026 08:38 |
| Increase, Hepatic intracellular active glucocorticoids | February 26, 2026 06:48 |
| Increase, Glucocorticoid receptor activation | February 12, 2026 07:24 |
| Increase, De novo lipogenesis | February 10, 2026 04:39 |
| Increase, Liver steatosis | February 11, 2026 05:41 |
| Increase, Hepatocellular lipotoxicity | February 10, 2026 04:40 |
| Increase, Endoplasmic reticulum stress | February 11, 2026 06:00 |
| Increase, Cell injury/death | May 27, 2024 07:23 |
| Increase, Kupffer cell activation | February 11, 2026 05:16 |
| Increase, Steatohepatitis | February 24, 2026 09:13 |
| Increase, Transforming growth factor-beta signaling | February 11, 2026 05:39 |
| Increase, Hepatic stellate cell activation | February 11, 2026 07:04 |
| Increase, Collagen accumulation | February 11, 2026 06:58 |
| Increase, Liver fibrosis | February 11, 2026 05:35 |
| Increase, Regenerative nodule formation | February 10, 2026 06:47 |
| Increase, Cirrhosis | February 11, 2026 07:34 |
| Increase, 11β-HSD1 activity leads to Increase, Hepatic intracellular active GC | February 24, 2026 08:32 |
| Increase, Hepatic intracellular active GC leads to Increase, GR activation | February 24, 2026 08:32 |
| Increase, GR activation leads to Increase, De novo lipogenesis | February 12, 2026 07:41 |
| Increase, De novo lipogenesis leads to Increase, Liver steatosis | February 11, 2026 05:41 |
| Increase, Liver steatosis leads to Increase, Hepatocellular lipotoxicity | February 10, 2026 08:59 |
| Increase, Hepatocellular lipotoxicity leads to Increase, ER stress | February 11, 2026 06:16 |
| Increase, ER stress leads to Cell injury/death | February 11, 2026 06:16 |
| Cell injury/death leads to Increase, Kupffer cell activation | November 29, 2016 19:54 |
| Increase, Kupffer cell activation leads to Increase, Steatohepatitis | February 10, 2026 09:00 |
| Increase, Steatohepatitis leads to Activation of TGF-β signaling | February 10, 2026 09:00 |
| Activation of TGF-β signaling leads to Increase, HSC activation | February 10, 2026 09:01 |
| Increase, HSC activation leads to Increase, Collagen accumulation | December 05, 2018 08:51 |
| Increase, Collagen accumulation leads to Increase, Liver fibrosis | December 05, 2018 08:52 |
| Increase, Liver fibrosis leads to Increase, Regenerative nodule formation | February 10, 2026 09:02 |
| Increase, Regenerative nodule formation leads to Increase, Cirrhosis | February 10, 2026 09:02 |
Abstract
This adverse outcome pathway (AOP) describes a mechanistic sequence linking altered glucocorticoid receptor (GR) signaling to the progression of metabolic dysfunction–associated steatotic liver disease (MASLD) through suppression of hepatic de novo lipogenesis (DNL) and subsequent endoplasmic reticulum (ER) stress. Disruption of GR signaling reduces coordinated lipogenic and lipid-buffering pathways, impairing the hepatocyte’s capacity to safely esterify and process fatty acids. This imbalance promotes hepatocellular lipotoxicity and lipid accumulation within the ER, triggering ER stress and maladaptive unfolded protein response signaling. Sustained ER stress leads to hepatocyte injury, inflammatory activation, and profibrotic signaling, including TGF-β–mediated hepatic stellate cell activation. These processes drive progression from steatosis to steatohepatitis (MASH), fibrosis, and cirrhosis. This AOP provides a biologically plausible and regulatory-relevant framework for identifying endocrine-disrupting chemicals (EDCs) that promote MASLD progression through GR-mediated disruption of hepatic lipid buffering and ER homeostasis.
AOP Development Strategy
Context
Although increased de novo lipogenesis is frequently associated with hepatic steatosis, adequate lipogenic capacity is also essential for maintaining ER and hepatocyte homeostasis. De novo lipogenesis supports triglyceride synthesis, lipid remodeling, and buffering of excess fatty acids, processes that limit the accumulation of toxic lipid intermediates within the ER. Suppression of DNL can therefore increase susceptibility to lipotoxic stress, particularly under conditions of ongoing lipid exposure.
Glucocorticoid receptor (GR) signaling regulates transcriptional programs involved in hepatic lipogenesis, fatty acid handling, and cellular stress responses. Altered GR signaling—due to dysregulation or chemical interference—can suppress DNL, disrupt ER lipid balance, and promote ER stress. This AOP was developed to capture this mechanistically distinct pathway linking GR perturbation to MASLD progression through ER stress rather than mitochondrial dysfunction.
Strategy
The AOP was developed using an expert-driven conceptual framework supported by targeted literature evaluation across endocrine signaling, hepatic lipid metabolism, ER stress biology, and chronic liver disease. Initial scoping identified reduced DNL as a plausible upstream disturbance capable of inducing ER stress independently of increased lipid influx or impaired lipid export.
Focused literature searches were conducted to identify evidence supporting:
-
GR regulation of hepatic lipogenic gene networks
-
Consequences of suppressed DNL on lipid buffering and ER lipid composition
-
Induction of ER stress by lipid imbalance and lipotoxic species
-
ER stress–mediated hepatocyte injury and inflammatory signaling
-
Fibrogenic pathways involving TGF-β signaling and hepatic stellate cell activation
Evidence from human studies, animal models, and mechanistic in vitro systems was prioritized, with emphasis on chronic perturbations relevant to endocrine disruption.
Summary of the AOP
Events:
Molecular Initiating Events (MIE)
Key Events (KE)
Adverse Outcomes (AO)
| Type | Event ID | Title | Short name |
|---|
| MIE | 2410 | Increase, 11β-Hydroxysteroid dehydrogenase type 1 activity | Increase, 11β-HSD1 activity |
| KE | 2412 | Increase, Hepatic intracellular active glucocorticoids | Increase, Hepatic intracellular active GC |
| KE | 122 | Increase, Glucocorticoid receptor activation | Increase, GR activation |
| KE | 1306 | Increase, De novo lipogenesis | Increase, De novo lipogenesis |
| KE | 2405 | Increase, Hepatocellular lipotoxicity | Increase, Hepatocellular lipotoxicity |
| KE | 1815 | Increase, Endoplasmic reticulum stress | Increase, ER stress |
| KE | 55 | Increase, Cell injury/death | Cell injury/death |
| KE | 134 | Increase, Kupffer cell activation | Increase, Kupffer cell activation |
| KE | 1271 | Increase, Transforming growth factor-beta signaling | Activation of TGF-β signaling |
| KE | 265 | Increase, Hepatic stellate cell activation | Increase, HSC activation |
| KE | 68 | Increase, Collagen accumulation | Increase, Collagen accumulation |
| KE | 2406 | Increase, Regenerative nodule formation | Increase, Regenerative nodule formation |
| AO | 459 | Increase, Liver steatosis | Increase, Liver steatosis |
| AO | 1489 | Increase, Steatohepatitis | Increase, Steatohepatitis |
| AO | 344 | Increase, Liver fibrosis | Increase, Liver fibrosis |
| AO | 2407 | Increase, Cirrhosis | Increase, Cirrhosis |
Relationships Between Two Key Events (Including MIEs and AOs)
| Title | Adjacency | Evidence | Quantitative Understanding |
|---|
Network View
Prototypical Stressors
Life Stage Applicability
Taxonomic Applicability
Sex Applicability
Overall Assessment of the AOP
This AOP is biologically plausible and supported by moderate empirical evidence demonstrating that suppression of hepatic lipogenesis can exacerbate ER stress and promote progressive liver injury. The sequence of key events reflects conserved cellular stress and fibrogenic mechanisms observed across mammalian species.
The AOP is particularly relevant for hazard identification and prioritization of chemicals that interfere with GR-regulated lipid homeostasis but may not induce classical steatogenic or insulin-resistant phenotypes. It complements other GR-mediated MASLD AOPs by highlighting ER stress as a downstream consequence of impaired lipid buffering capacity.
Domain of Applicability
-
Taxa: Mammals (humans and laboratory rodents)
-
Life stage: Primarily adolescents and adults
-
Sex: Applicable to both sexes; sex-dependent modulation of lipogenic and stress responses may occur
-
Biological context: Chronic endocrine perturbation, altered lipid handling, metabolic stress
This AOP is not intended to describe acute liver toxicity and is most applicable to chronic exposure scenarios.
Essentiality of the Key Events
Evidence supporting the essentiality of the key events includes:
-
Altered GR signaling: Experimental modulation of GR activity alters hepatic lipogenic programs and ER homeostasis.
-
Reduced de novo lipogenesis: Suppression of DNL limits lipid buffering capacity, increasing susceptibility to ER lipid overload and stress.
-
ER stress: Attenuation of ER stress pathways reduces hepatocyte injury, inflammation, and disease severity in MASLD models.
-
Inflammatory and fibrogenic activation: Inhibition of Kupffer cell activation, hepatic stellate cell activation, or TGF-β signaling mitigates fibrosis progression.
These findings support the causal role of each KE in driving downstream MASLD outcomes.
Evidence Assessment
Across the KERs in this AOP:
-
Biological plausibility is strong, based on established roles of DNL in maintaining ER lipid balance and hepatocyte homeostasis.
-
Empirical support is moderate, with increasing evidence linking reduced lipogenesis to ER stress and liver injury.
-
Quantitative understanding is limited, particularly regarding thresholds at which reduced DNL transitions from adaptive to maladaptive responses.
Overall, the weight of evidence supports confidence in the pathway for regulatory-relevant applications.
Known Modulating Factors
| Modulating Factor (MF) | Influence or Outcome | KER(s) involved |
|---|---|---|
| Dietary fatty acid load | Modulates reliance on DNL for lipid buffering | DNL ↓ → ER stress |
| Insulin signaling status | Influences lipogenic gene expression | GR signaling → DNL |
| ER chaperone capacity | Alters resilience to lipid-induced ER stress | ER stress → cell injury |
| Inflammatory milieu | Amplifies hepatocyte injury and fibrogenesis | Cell injury → fibrosis |
Quantitative Understanding
Quantitative data exist for individual links between reduced DNL, altered lipid composition, and ER stress marker induction. However, quantitative integration across downstream inflammatory and fibrotic events remains limited. Accordingly, this AOP is best applied qualitatively or semi-quantitatively.
Considerations for Potential Applications of the AOP (optional)
This AOP may support:
-
Identification of GR-modulating chemicals that impair hepatic lipid buffering capacity
-
Inclusion of ER stress endpoints in MASLD-relevant testing strategies
-
Complementary assessment of ER-centered mechanisms alongside mitochondrial stress pathways
-
Construction of AOP networks capturing multiple GR-mediated routes to MASLD progression