
This AOP is licensed under the BY-SA license. This license allows reusers to distribute, remix, adapt, and build upon the material in any medium or format, so long as attribution is given to the creator. The license allows for commercial use. If you remix, adapt, or build upon the material, you must license the modified material under identical terms.
AOP: 212
Title
Histone deacetylase inhibition leading to testicular atrophy
Short name
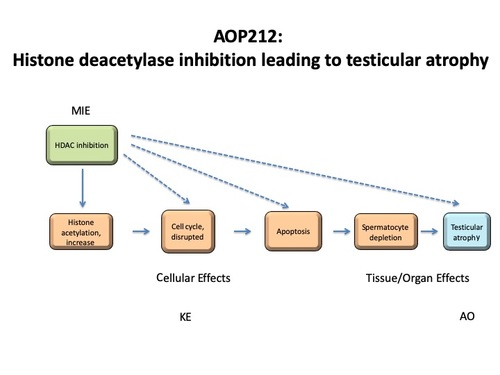
Graphical Representation
Point of Contact
Contributors
- Shihori Tanabe
Coaches
OECD Information Table
| OECD Project # | OECD Status | Reviewer's Reports | Journal-format Article | OECD iLibrary Published Version |
|---|---|---|---|---|
| 1.52 | WPHA/WNT Endorsed | Scientific Review | Adverse Outcome Pathway on histone deacetylase inhibition leading to testicular atrophy |
This AOP was last modified on April 18, 2024 03:45
Revision dates for related pages
| Page | Revision Date/Time |
|---|---|
| Histone deacetylase inhibition | July 14, 2022 16:18 |
| Histone acetylation, increase | July 28, 2021 00:10 |
| Cell cycle, disrupted | June 30, 2021 02:56 |
| Apoptosis | May 31, 2025 08:50 |
| Testicular atrophy | July 01, 2021 01:55 |
| Spermatocyte depletion | July 28, 2021 02:14 |
| Histone deacetylase inhibition leads to Histone acetylation, increase | August 05, 2021 19:32 |
| Histone acetylation, increase leads to Cell cycle, disrupted | July 01, 2021 03:28 |
| Cell cycle, disrupted leads to Apoptosis | July 28, 2021 01:28 |
| Histone deacetylase inhibition leads to Cell cycle, disrupted | July 28, 2021 01:32 |
| Apoptosis leads to Spermatocyte depletion | July 28, 2021 01:31 |
| Histone deacetylase inhibition leads to Apoptosis | July 01, 2021 21:38 |
| Spermatocyte depletion leads to Testicular atrophy | July 28, 2021 00:45 |
| Histone deacetylase inhibition leads to Spermatocyte depletion | July 01, 2021 21:43 |
| Histone deacetylase inhibition leads to Testicular atrophy | July 28, 2021 01:33 |
| Methoxyacetic acid | January 21, 2018 20:38 |
| Butyrate | January 21, 2018 20:39 |
| Trichostatin A | January 21, 2018 20:39 |
| Valproate | November 29, 2016 18:42 |
Abstract
Testicular toxicity is of interest for human health risk assessment especially in terms of reproductive and developmental toxicity, however, the testicular toxicity has not been fully elucidated. Histone deacetylase inhibitors (HDIs) are approved as anti-cancer drugs since HDIs have apoptotic effects in cancer cells. HDIs include short-chain fatty acids, hydroxamic acids, benzamides, and epoxides. The intracellular mechanisms of induction of the spermatocyte apoptosis by HDIs are suggested as histone deacetylase (HDAC) inhibition as MIE, histone acetylation increase, disrupted cell cycle, apoptosis, and spermatocyte depletion as KEs. The adverse outcome has been defined as testicular atrophy. The HDIs inhibit deacetylation of the histone, leading to an increase in histone acetylation. The apoptosis induced by the disrupted cell cycle leads to spermatocyte depletion and testis atrophy. This AOP may be one of the pathways induced by HDIs, which suggests the pathway networks of protein hyperacetylations.
[Abbreviation] AOP: adverse outcome pathway, HDAC: histone deacetylase, HDI: HDAC inhibitor, KE: key event, MIE: molecular initiating event, MAA: 2-Methoxyacetic acid, or Methoxyacetic acid
AOP Development Strategy
Context
Strategy
Summary of the AOP
Events:
Molecular Initiating Events (MIE)
Key Events (KE)
Adverse Outcomes (AO)
| Type | Event ID | Title | Short name |
|---|
| MIE | 1502 | Histone deacetylase inhibition | Histone deacetylase inhibition |
| KE | 1503 | Histone acetylation, increase | Histone acetylation, increase |
| KE | 1505 | Cell cycle, disrupted | Cell cycle, disrupted |
| KE | 1262 | Apoptosis | Apoptosis |
| KE | 1515 | Spermatocyte depletion | Spermatocyte depletion |
| AO | 1506 | Testicular atrophy | Testicular atrophy |
Relationships Between Two Key Events (Including MIEs and AOs)
| Title | Adjacency | Evidence | Quantitative Understanding |
|---|
| Histone deacetylase inhibition leads to Histone acetylation, increase | adjacent | High | Moderate |
| Histone acetylation, increase leads to Cell cycle, disrupted | adjacent | Moderate | Moderate |
| Cell cycle, disrupted leads to Apoptosis | adjacent | Moderate | Moderate |
| Apoptosis leads to Spermatocyte depletion | adjacent | High | Not Specified |
| Spermatocyte depletion leads to Testicular atrophy | adjacent | High | Not Specified |
| Histone deacetylase inhibition leads to Cell cycle, disrupted | non-adjacent | High | Moderate |
| Histone deacetylase inhibition leads to Apoptosis | non-adjacent | Moderate | Moderate |
| Histone deacetylase inhibition leads to Spermatocyte depletion | non-adjacent | Moderate | Moderate |
| Histone deacetylase inhibition leads to Testicular atrophy | non-adjacent | Moderate | Moderate |
Network View
Prototypical Stressors
Life Stage Applicability
| Life stage | Evidence |
|---|---|
| Adult, reproductively mature | High |
Taxonomic Applicability
Sex Applicability
| Sex | Evidence |
|---|---|
| Male | High |
Overall Assessment of the AOP

|
1. Support for Biological Plausibility of KERs |
|
|
MIE => KE1: Histone deacetylase inhibition leads to histone acetylation increase |
Biological Plausibility of the MIE => KE1 is high. Rationale: Upon the inhibition of HDAC by HDIs, the acetylation of lysine in histone remains and it leads to transcriptional activation or repression, changes in DNA replication, and DNA damage repair. The activity of histone acetyltransferase (HAT) in testis nuclear protein was increased with MAA addition [Wade et al., 2008]. |
|
KE1 => KE2: Histone acetylation, increase leads to cell cycle, disrupted |
Biological Plausibility of the KE1 => KE2 is moderate. Rationale: Gene transcription is regulated by histone acetylation [Struhl, 1998]. Acetylation of histones neutralizes the positive charge of the histones. Thus, less compacted DNA can be bound more easily by transcription factors and transcribed. In the models proposed for the relationship between histone acetylation and transcription, histone acetylation can be untargeted and occur at both promoter and non-promoter regions, targeted generally to promoter regions, or targeted to specific promoters by gene-specific activator proteins [Richon et al., 2000; Struhl, 1998]. |
|
KE2 => KE3: Cell cycle, disrupted leads to apoptosis |
Biological Plausibility of the KE2 => KE3 is moderate. Rationale: Prolonged cell cycle arrest will lead to either senescence or apoptosis. Especially for fast-dividing and still differentiating cells, such an arrest will most certainly induce apoptosis as the normal cellular program cannot be followed. |
|
KE3 => KE4: Apoptosis leads to spermatocyte depletion |
Biological Plausibility of the KE3 => KE4 is moderate. Rationale: During development and in tissue homeostasis, apoptosis is needed to control organ size. If apoptosis is induced at a higher rate, one can assume it leading to atrophy of the target organ. Especially when target organ/target cells are fast replicating, abnormal levels of apoptosis will lead to depletion. |
|
KE4 => AO: Spermatocyte depletion leads to testicular atrophy |
Biological Plausibility of the KE4 => AO is moderate. Rationale: Spermatocyte depletion is one of the main characteristics of testicular atrophy. |
|
2. Support for Essentiality of KEs |
|
|
KE2: Cell cycle, disrupted |
The essentiality of the KE2 is moderate. The rationale for the Essentiality of KEs in the AOP: HDAC1-deficient embryonic stem cells showed reduced proliferation rates, which correlates with decreased cyclin-associated kinase activities and elevated levels of the cyclin-dependent kinase inhibitor 1A, a cell cycle regulator p21 [Lagger et al., 2002]. Loss of HDAC1 leads to significantly reduced overall deacetylase activity, hyperacetylation of a subset of histones H3 and H4 [Lagger et al., 2002]. |
|
3. Empirical Support for KERs |
|
|
MIE => KE1: Histone deacetylase inhibition leads to histone acetylation, increase |
Empirical Support of the MIE => KE1 is high. Rationale: HDAC inhibitors increase histone acetylation in the brain [Schroeder et al., 2013]. The major empirical evidence came from the incubation of cell culture cells with small molecule compounds that inhibit HDAC enzymes followed by western blots or acid urea gel analysis. The first evidence was shown by Riggs et al. who showed that incubation of HeLa cells with n-butyrate leads to an accumulation of acetylated histone proteins [Riggs et al., 1977]. Later, it was shown that n-butyrate specifically increases the acetylation of histone by the incorporation of radioactive [3H]acetate and analysis of the histones on acid urea gels that allow the detection of acetylated histones [Cousens et al., 1979]. TSA was shown to be an HDAC inhibitor by acid urea gel analysis in 1990 [Yoshida et al., 1990] and good evidence for VPA as an HDAC inhibitor in vitro and in vivo was shown using acetyl-specific antibodies and western blot [Gottlicher et al., 2001]. |
|
KE1 => KE2: Histone acetylation, increase leads to cell cycle, disrupted |
Empirical Support of the KE1 => KE2 is moderate. Rationale: Increase in histone acetylation by HDAC inhibition induces the cell cycle regulator expression and inhibits progression through the cell cycle. Histone acetylation regulates the gene transcriptional mechanism [Struhl, 1998]. Acetylation of histones promotes the RNA polymerase reaction [Allfrey et al., 1964; Pogo et al., 1966]. Since several results supported a model in which increased histone acetylation is targeted to a specific gene and occurs throughout the entire genome, not just the promoter regions, histone acetylation may lead to gene transcription of the cell cycle regulator [Richon et al., 2000]. |
|
KE2 => KE3: Cell cycle, disrupted leads to apoptosis |
Empirical Support of the KE2 => KE3 is moderate. Rationale: Cell cycle arrests such as G1 arrest and G1/S arrest are observed in apoptosis [Li et al., 2012; Dong et al., 2010]. microRNA-1 and microRNA-206 repress CCND2, while microRNA-29 represses CCND2 and induces G1 arrest and apoptosis in rhabdomyosarcoma [Li et al., 2012]. |
|
KE3 => KE4: Apoptosis leads to spermatocyte depletion |
Empirical Support of the KE3 => KE4 is high. Rationale: microRNA-21 regulates the spermatogonial stem cell homeostasis, in which suppression of microRNA-21 with anti-miR-21 oligonucleotides led to apoptosis of spermatogonial stem cell-enriched germ cell cultures and the decrease in the number of spermatogonial stem cells [Niu et al., 2011]. |
|
KE4 => AO: Spermatocyte depletion leads to testicular atrophy |
Empirical Support of the KE4 => AO is high. Rationale: The testicular atrophy seen in 2-methoxyethanol (2-ME), or its major metabolite MAA, treated rats in vivo and in human, and rat in vitro culture was associated with spermatocyte depletion [Beattie et al., 1984]. |
Domain of Applicability
The AOP is applicable to the reproductively mature males in rats, mice and humans. The administration route or doses of HDAC inhibitors may affect the intensity of the toxicity.
Essentiality of the Key Events
| Key Event | Direct/Indirect Evidence |
| MIE: Histone deacetylase inhibition | HDAC inhibition induced testicular toxicity including testis atrophy [Miller et al., 1982]. HDAC inhibition in cell culture resulted in testicular toxicity including germ cell apoptosis and cell morphology change [Li et al., 1996]. |
| KE1: Histone acetylation, increase | The HDAC inhibition induced cell death in spermatocytes in both rat and human seminiferous tubules [Li et al., 1996]. |
| KE2: Cell cycle, disrupted | In HDAC1-/- fibroblast lines, an increase in the number of cells in the G1 phase and a decrease in the number of cells in the S phase were observed, which indicates the importance of HDAC inhibition in cell cycle regulation [Zupkovitz et al., 2010]. |
| KE3: Apoptosis | HDAC inhibition leads to cell death through the apoptotic pathways [Falkenberg et al., 2014]. |
| KE4: Spermatocyte depletion | The HDAC inhibition induced cell death in spermatocytes in both rat and human seminiferous tubules [Li et al., 1996]. The HDAC inhibitor treatment resulted in degeneration in spermatocytes in rat seminiferous tubules [Li et al., 1996]. The HDAC inhibition induced germ cell apoptosis in human testicular tissues [Li et al., 1996]. |
Evidence Assessment
Biological plausibility, coherence, and consistency of the experimental evidence
The available data supporting the AOP are logical, coherent, and consistent with established biological knowledge, whereas there are possibilities for alternative pathways.
Alternative mechanism(s) that logically present themselves and the extent to which they may distract from the postulated AOP
There are some other important apoptotic pathways that are involved in cell death, as well as other important spermatocyte signaling or mechanism influences testicular toxicity.
- p53 pathway
The study in which in vivo administration of trichostatin A (TSA), an HDI, in mice resulted in male meiosis impairment showed the involvement of p53-noxa-caspase-3 apoptotic pathway in TSA-induced spermatocyte apoptosis [Fenic et al., 2008]. Another study showed that MAA-induced up-regulation of p21 expression is mediated through histone hyperacetylation and independent of p53/p63/p73 [Parajuli et al., 2014].
- NF-kappaB pathway
The present AOP focuses on the p21 pathway leading to apoptosis, however, alternative pathways such as NF-kappaB signaling pathways may be involved in the apoptosis of spermatocytes [Wang et al., 2017].
- Communication with Sertoli cells
The present AOP focuses on testicular atrophy by HDAC inhibition-induced apoptosis in spermatocytes, however, the signaling in Sertoli cells may be involved in testicular atrophy. Sertoli cell secretes GDNF, FGF2, CXCL12, or Ccl9 molecules, which results in the activation of RET, FGFR, CXCR4, or CCR1 signaling in spermatogonial stem cells, respectively [Chen and Liu, 2015].
- Decrease in deoxynucleotide pool by MAA
MAA induces a decrease in the deoxynucleotide pool, resulting in apoptosis, which may be an alternative pathway other than the p21-mediated pathway [Yamazoe et al., 2015]. Inhibition of 5,10-CH2-THF production by MAA may decrease deoxynucleotide pool in spermatocytes [Yamazoe et al., 2015].
- Spermatocyte depletion by necrosis
Spermatocyte may be decreased by necrosis. Cell death mechanisms other than apoptosis, such as necrosis, may be considered for spermatocyte depletion.
Known Modulating Factors
| Modulating Factor (MF) | Influence or Outcome | KER(s) involved |
|---|---|---|
Quantitative Understanding
Concordance of dose-response relationships
This is a quantitative description of dose-response relationships from MIE to AOP. But some KE relationships individually are not fully supported with dose-response relationships, while there is empirical evidence to support that a change in KEup leads to an appropriate change in the respective KEdown.
Temporal concordance among the key events and adverse outcome
Temporal concordance between MIE and AOP has been described with in vivo experimental data. Empirical evidence shows temporal concordance between MIE and the individual KEs, however, the temporal concordance among the individual KEs and AO is not fully elucidated.
Strength, consistency, and specificity of association of adverse outcome and initiating event
The scientific evidence on the linkage between MIE and AO has been described.
The quantitative understanding of the AOP in terms of indirect relations between HDAC inhibition and testicular atrophy was examined in in vivo experiments [Foster et al., 1983; Miller et al., 1982].
Considerations for Potential Applications of the AOP (optional)
The AOP may be useful in the risk assessment on several types of HDI molecules including anti-cancer drugs, as well as other types of chemicals, biocides, or pesticides. HDAC inhibitors nowadays have been utilized as therapeutics for cancer or neurology disease, and the adverse effects of HDAC inhibitors should be evaluated. This AOP elucidating the pathway from HDAC inhibition to testicular atrophy may provide important insights into the potential toxicity of HDAC inhibitors. It also provides a basis for the HDAC inhibition-induced epigenetic alteration and cell death. HDAC inhibitors such as rocilinostat/ricolinostat are clinically evaluated as anti-cancer drugs in clinical trials [Yee et al., 2016]. The AOP may be useful for the risk assessment of chemicals, since possible applications of HDAC inhibitors include the enhancement of salinity tolerance to increase agricultural sustainability. Other potential applications of the AOP include the risk assessment of biocides or pesticides, considering that HDAC inhibitors are being investigated as insecticides or amoebicides [Bagnall et al., 2017; Lee et al., 2020].
References
Allfrey, V. et al. (1964), "Acetylation and methylation of histones and their possible role in the regulation of RNA synthesis", Proc Natl Acad Sci 51:786-794
Bagnall, N.H. et al. (2017), "Insecticidal activities of histone deacetylase inhibitors against a dipteran parasite of sheep, Lucilia cuprina", Int J Parasitology: Drugs Drug Resistance 7(1):51–60 https://doi.org/10.1016/j.ijpddr.2017.01.001
Beattie, P.J. et al. (1984), "The effect of 2-methoxyethanol and methoxyacetic acid on Sertoli cell lactate production and protein synthesis in vitro", Toxicol Appl Pharmacol 76:56-61
Chen, S. and Liu, Y. (2015), "Regulation of spermatogonial stem cell self-renewal and spermatocyte meiosis by Sertoli cell signaling", Reproduction 149:R159-R167
Cousens, L.S., et al. (1979), "Different accessibilities in chromatin to histone acetylase", J Biol Chem 254:1716-1723
Dong, Q. et al. (2010), "microRNA let-7a inhibits proliferation of human prostate cancer cells in vitro and in vivo by targeting E2F2 and CCND2", PLoS One 5:e10147
Fenic, I. et al. (2008), "In vivo application of histone deacetylase inhibitor trichostatin-A impairs murine male meiosis", J Andro 29:172-185
Foster, P.M. et al. (1983), "Testicular toxicity of ethylene glycol monomethyl and monoethyl ethers in the rat", Toxicol Appl Pharmacol 69:385-39
Gottlicher, M. et al. (2001), "Valproic acid defines a novel class of HDAC inhibitors inducing differentiation of transformed cells", EMBO J 20:6969-6978
Lagger, G. et al. (2002), "Essential function of histone deacetylase 1 in proliferation control and CDK inhibitor repression", EMBO J 21:2672-2681
Lee, H-A. et al. (2020), "Application of histone deacetylase inhibitors MPK472 and KSK64 as a potential treatment option for Acanthamoeba keratitis" Antimicrob Agents Chemother 64:e01506-20 https://doi.org/10.1128/AAC.01506-20
Li, L. et al. (2012), "Downregulation of microRNAs miR-1, -206 and -29 stabilizes PAX3 and CCND2 expression in rhabdomyosarcoma", Lab Invest 92:571-583
Li, L.H. et al. (1996), "2-Methoxyacetic acid (MAA)-induced spermatocyte apoptosis in human and rat testes: an in vitro comparison", J Androl 17:538-549
Miller, R.R. et al. (1982), "Toxicity of methoxyacetic acid in rats", Fundam Appl Toxicol 2:158-160
Niu, Z. et al. (2011), "microRNA-21 regulates the self-renewal of mouse spermatogonial stem cells", Proc Natl Acad Sci 108:12740-12745
Parajuli, K.R. et al. (2014), "Methoxyacetic acid suppresses prostate cancer cell growth by inducing growth arrest and apoptosis", Am J Clin Exp Urol 2:300-312
Pogo, B. et al. (1966), "RNA synthesis and histone acetylation during the course of gene activation in lymphocytes", Proc Natl Acad Sci 55:805-812
Richon, V.M. et al. (2000), "Histone deacetylase inhibitor selectively induces p21WAF1 expression and gene-associated histone acetylation", Proc Natl Acad Sci 97:10014-10019
Riggs, M.G. et al. (1977), "N-butyrate causes histone modification in HeLa and friend erythroleukaemia cells", Nature 268:462-464
Schroeder, F.A. et al. (2013), "A selective HDAC 1/2 inhibitor modulates chromatin and gene expression in brain and alters mouse behavior in two mood-related tests", PLoS One 8:e71323
Struhl, K. (1998), "Histone acetylation and transcriptional regulatory mechanisms", Gene Dev 12:599-606
Wade, M.G. et al. (2008), "Methoxyacetic acid-induced spermatocyte death is associated with histone hyperacetylation in rats", Biol Reprod 78:822-831
Wang, C. et al. (2017), "CD147 regulates extrinsic apoptosis in spermatocytes by modulating NFkB signaling pathways", Oncotarget 8:3132-3143
Yamazoe, Y. et al. (2015), "Embryo- and testicular-toxicities of methoxyacetate and the related: a review on possible roles of one-carbon transfer and histone modification", Food Safety 3:92-107
Yee, A.J. et al. (2016), "Ricolinostat plus lenalidomide, and dexamethasone in relapsed or refractory multiple myeloma: a multicentre phase 1b trial", Lancet Oncol 17(11):1569-1578 https://doi.org/10.1016/s1470-2045(16)30375-8
Yoshida, M. et al. (1990), "Potent and specific inhibition of mammalian histone deacetylase both in vivo and in vitro trichostatin A", J Biol Chem 265:17174-17179
Zupkovitz, G. et al. (2010), "The cyclin-dependent kinase inhibitor p21 is a crucial target for histone deacetylase 1 as a regulator of cellular proliferation", Mol Cell Biol 30:1171-1181