
This AOP is licensed under the BY-SA license. This license allows reusers to distribute, remix, adapt, and build upon the material in any medium or format, so long as attribution is given to the creator. The license allows for commercial use. If you remix, adapt, or build upon the material, you must license the modified material under identical terms.
AOP: 298
Title
Increase in reactive oxygen species (ROS) leading to human treatment-resistant gastric cancer
Short name
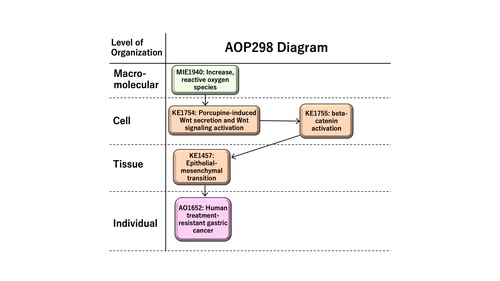
Graphical Representation
Point of Contact
Contributors
- Shihori Tanabe
Coaches
- Edward Perkins
- You Song
OECD Information Table
| OECD Project # | OECD Status | Reviewer's Reports | Journal-format Article | OECD iLibrary Published Version |
|---|---|---|---|---|
| 1.58 | Under Review | Scientific Review | Adverse Outcome Pathway 298: Increase in Reactive Oxygen Species Leading to Human Treatment-Resistant Gastric Cancer |
This AOP was last modified on August 14, 2025 22:12
Revision dates for related pages
| Page | Revision Date/Time |
|---|---|
| Treatment-resistant gastric cancer | June 12, 2025 01:33 |
| Increase, porcupine-induced Wnt secretion and Wnt signaling activation | June 04, 2025 02:36 |
| beta-catenin activation | November 25, 2022 01:14 |
| Epithelial Mesenchymal Transition | April 24, 2024 00:44 |
| Increase, Reactive oxygen species | June 12, 2025 01:27 |
| Increase, ROS leads to Increase, porcupine-induced Wnt secretion and Wnt signaling activation | July 22, 2025 01:21 |
| Increase, porcupine-induced Wnt secretion and Wnt signaling activation leads to beta-catenin activation | November 09, 2021 01:23 |
| beta-catenin activation leads to EMT | April 23, 2024 17:35 |
| EMT leads to Resistant gastric cancer | April 23, 2024 17:37 |
| Wnt | May 29, 2019 03:59 |
| WNT2 | May 29, 2019 03:59 |
| Porcupine | January 19, 2020 21:19 |
| Wntless | January 19, 2020 21:20 |
| Ionizing Radiation | May 07, 2019 12:12 |
| ferric nitrilotriacetate | May 27, 2020 02:40 |
Abstract
This AOP entitled “Increase in reactive oxygen species (ROS) leading to human treatment-resistant gastric cancer” consists of MIE as KE1115 “Increase, ROS” followed by KE1 as KE1754 “porcupine-induced Wnt secretion and Wnt signaling activation,” KE2 as KE1755 “beta-catenin activation,” KE3 as KE1457 “epithelial-mesenchymal transition (EMT),” and AO as KE1651 “human treatment-resistant gastric cancer.” ROS has multiple roles in disease, such as the development and progression of cancer or apoptotic induction, causing anti-tumor effects. In this AOP, we focus on sustained levels of chronic reactive oxygen species (ROS) in inducing therapy resistance in human gastric cancer. Epithelial-mesenchymal transition (EMT), a cellular phenotypic change from epithelial to mesenchymal-like features, demonstrates cancer stem cell-like characteristics in human gastric cancer. EMT is induced by Wnt/beta-catenin signaling, providing the rationale to have Wnt secretion and beta-catenin activation as KE1 and KE2 on the AOP, respectively. The AOP might be useful for the development of anti-cancer drugs or the prediction of adverse effects of therapeutics, which are of possible regulatory relevance.
AOP Development Strategy
Context
Strategy
The references related to reactive oxygen species and human treatment-resistant gastric cancer were searched in PubMed and internet.
Gene expression analysis was performed in diffuse- and intestinal-type gastric cancer to elucidate mechanisms of epithelial-mesenchymal transition in human treatment-resistant gastric cancer.
Summary of the AOP
Events:
Molecular Initiating Events (MIE)
Key Events (KE)
Adverse Outcomes (AO)
| Type | Event ID | Title | Short name |
|---|
| MIE | 1115 | Increase, Reactive oxygen species | Increase, ROS |
| KE | 1754 | Increase, porcupine-induced Wnt secretion and Wnt signaling activation | Increase, porcupine-induced Wnt secretion and Wnt signaling activation |
| KE | 1755 | beta-catenin activation | beta-catenin activation |
| KE | 1457 | Epithelial Mesenchymal Transition | EMT |
| AO | 1651 | Treatment-resistant gastric cancer | Resistant gastric cancer |
Relationships Between Two Key Events (Including MIEs and AOs)
| Title | Adjacency | Evidence | Quantitative Understanding |
|---|
| Increase, ROS leads to Increase, porcupine-induced Wnt secretion and Wnt signaling activation | adjacent | Moderate | Moderate |
| Increase, porcupine-induced Wnt secretion and Wnt signaling activation leads to beta-catenin activation | adjacent | Moderate | Moderate |
| beta-catenin activation leads to EMT | adjacent | Moderate | Moderate |
| EMT leads to Resistant gastric cancer | adjacent | Moderate | Moderate |
Network View
Prototypical Stressors
Life Stage Applicability
| Life stage | Evidence |
|---|---|
| All life stages | High |
Taxonomic Applicability
| Term | Scientific Term | Evidence | Link |
|---|---|---|---|
| Homo sapiens | Homo sapiens | High | NCBI |
Sex Applicability
| Sex | Evidence |
|---|---|
| Unspecific | High |
Overall Assessment of the AOP

|
1. Support for Biological Plausibility of KERs |
|
|
MIE => KE1: Increase, ROS leads to porcupine-induced Wnt secretion and Wnt signaling activation |
Biological Plausibility of the MIE => KE1 is moderate. Rationale: Sustained ROS caused by/causes DNA damage, which will alter several signaling pathways, including Wnt signaling. ROS stimulate inflammatory factor production and Wnt/beta-catenin signaling. Macrophages accumulate in injured tissue to recover the tissue damage, which may be followed by porcupine-induced Wnt secretion (Vallée & Lecarpentier, 2018). |
|
KE1 => KE2: Porcupine-induced Wnt secretion and Wnt signaling activation leads to beta-catenin activation |
Biological Plausibility of the KE1 => KE2 is moderate. Rationale: Secreted Wnt ligand stimulates Wnt/beta-catenin signaling, in which beta-catenin is activated. Wnt ligand binds to Frizzled receptor, which leads to GSK3b inactivation. GSK3b inactivation leads to beta-catenin dephosphorylation, which avoids the ubiquitination of the beta-catenin and stabilizes the beta-catenin (Clevers & Nusse, 2012). |
|
KE2 => KE3: beta-catenin activation leads to Epithelial-mesenchymal transition (EMT) |
Biological Plausibility of the KE2 => KE3 is moderate. Rationale: beta-catenin activation, of which mechanisms include the stabilization of the dephosphorylated beta-catenin and translocation of beta-catenin into the nucleus, induces the formation of beta-catenin-TCF complex and transcription of transcription factors such as Snail, Zeb, and Twist (Clevers & Nusse, 2012) (Ahmad et al., 2012; Pearlman et al., 2017; Sohn et al., 2019; Yang W et al., 2019). EMT-related transcription factors, including Snail, ZEB, and Twist, are up-regulated in cancer cells (Diaz et al., 2014). The transcription factors such as Snail, ZEB, and Twist bind to the E-cadherin (CDH1) promoter and inhibit the CDH1 transcription via the consensus E-boxes (5’-CACCTG-3’ or 5’-CAGGTG-3’), which leads to EMT (Diaz et al., 2014). |
|
KE3 => AO: Epithelial-mesenchymal transition (EMT) leads to treatment-resistant gastric cancer |
Biological Plausibility of the KE3 => AO is moderate. Rationale: Some populations of cells exhibiting EMT demonstrate the feature of cancer stem cells (CSCs), which are related to cancer malignancy (Shibue & Weinberg, 2017; Tanabe, 2015a, 2015b; Tanabe et al., 2015). EMT phenomenon is related to cancer metastasis and cancer therapy resistance (Smith & Bhowmick, 2016; Tanabe, 2013). The increase in expression of enzymes that degrade the extracellular matrix components and the decrease in adhesion to the basement membrane in EMT induce the cell to escape from the basement membrane and metastasis (Smith & Bhowmick, 2016). Morphological changes observed during EMT are associated with therapy resistance (Smith & Bhowmick, 2016). |
|
2. Support for essentiality of KEs |
|
|
MIE: Increase, ROS |
Essentiality of the MIE is high. Rationale for Essentiality of the MIE in the AOP: Sustained ROS contributes to the initiation and development of human gastric cancer (Gu et al., 2018). |
|
KE1: Porcupine-induced Wnt secretion and Wnt signaling activation |
Essentiality of the KE1 is moderate. Rationale for Essentiality of the KE1 in the AOP: The Wnt signaling activation is essential for the subsequent beta-catenin activation and cancer resistance (Tanabe, 2018). |
|
KE2: beta-catenin activation |
Essentiality of the KE2 is moderate. Rationale for Essentiality of the KE2 in the AOP: beta-catenin activation is essential for the Wnt-induced cancer resistance (Tanabe, 2018). |
|
KE3: Epithelial-mesenchymal transition (EMT) |
Essentiality of the KE3 is moderate. Rationale for Essentiality of the KE3 in the AOP: EMT is essential for the Wnt-induced cancer promotion and acquisition of resistance to anti-cancer drugs (Tanabe, 2018; Tanabe et al, 2020a, 2020b, 2023). |
Domain of Applicability
The AOP298 applies to Homo sapiens (human), all life stages, and both male and female.
Essentiality of the Key Events
Sustained ROS contributes to the initiation and development of human gastric cancer (Gu et al., 2018).
Wnt signaling is involved in cancer malignancy (Tanabe, 2018).
Upon stimulation with Wnt ligand to the Frizzled receptor, Wnt/beta-catenin signaling is activated. Wnt/beta-catenin consists of GSK3 beta inactivation, beta-catenin activation, and up-regulation of transcription factors such as Zeb, Twist, and Snail. The transcription factors Zeb, Twist and Snail relate to the activation of EMT-related genes. EMT is regulated with various gene networks (Tanabe, 2015c, Tanabe et al, 2020a, 2020b).
| Event | Direct Evidence | Indirect Evidence | No experimental evidence |
| MIE: Increase, ROS | *** | * | |
| KE1: Increase, porcupine-induced Wnt secretion and Wnt signaling activation | * | ** | |
| KE2: beta-catenin activation | * | ** | |
| KE3: Epithelial-Mesenchymal Transition | * | ** |
Evidence Assessment
The Wnt signaling promotes EMT and cancer malignancy in colorectal cancer (Lazarova & Bordonaro, 2017). Although the potential pathways other than Wnt signaling exist in EMT induction and the mechanism underlaid cancer malignancy, Wnt signaling is one of the main pathways to induce EMT and cancer malignancy (Polakis, 2012).
| MIE => KE1: Increases in cellular ROS leads to porcupine-induced Wnt secretion and Wnt signaling activation | Empirical Support of the MIE => KE1 is moderate. Rationale: Production of ROS and DNA double-strand break cause the tissue damages (Gao et al., 2019). ROS-related signaling induces Wnt/b-catenin pathway activation (Pérez et al., 2017). |
| KE1 => KE2: Porcupine-induced Wnt secretion and Wnt signaling activation leads to beta-catenin activation | Empirical Support of the KE1 => KE2 is moderate. Rationale: Dishevelled (DVL), a positive regulator of Wnt signaling, form the complex with FZD and lead to trigger the Wnt signaling together with Wnt coreceptor low-density lipoprotein (LDL) receptor-related protein 6 (LRP6) (Clevers & Nusse, 2012; Jiang et al., 2015). Wnt binds to FZD and activate the Wnt signaling (Clevers & Nusse, 2012; Janda et al., 2012; Nile et al., 2017). Wnt binding towards FZD induce the formation of the protein complex with LRP5/6 and DVL, leading to the down-stream signaling activation including beta-catenin (Clevers & Nusse, 2012). |
| KE2 => KE3: beta-catenin activation leads to Epithelial-mesenchymal transition (EMT) | Empirical Support of the KE2 => KE3 is moderate. Rationale: The inhibition of c-MET, which is overexpressed in diffuse-type gastric cancer, induced increase in phosphorylated b-catenin, decrease in b-catenin and Snail (Sohn et al., 2019). The garcinol, that has anti-cancer effect, increases phosphorylated beta-catenin, decreases b-catenin and ZEB1/ZEB2, and inhibit EMT (Ahmad et al., 2012). The inhibition of sortilin by AF38469 (a sortilin inhibitor) or small interference RNA (siRNA) results in decrease in b-catenin and Twist expression in human glioblastoma cells (Yang W. et al., 2019). Histone deacetylase inhibitors effect on EMT-related transcription factors including ZEB, Twist and Snail (Wawruszak et al., 2019). Snail and Zeb induces EMT and suppress E-cadherin (CDH1) (Batlle et al., 2000; Diaz et al., 2014; Peinado et al., 2007). |
| KE3 => AO: Epithelial-mesenchymal transition (EMT) leads to Treatment-resistant gastric cancer | Empirical Support of the KE3 => AO is moderate. Rationale: EMT activation induces the expression of multiple members of the ATP-binding cassette (ABC) transporter family, which results in doxorubicin resistance (Saxena et al., 2011; Shibue & Weinberg, 2017). TGFb-1 induced EMT results in the acquisition of cancer stem cell (CSC) like properties (Pirozzi et al., 2011; Shibue & Weinberg, 2017). Snail-induced EMT induces the cancer metastasis and resistance to dendritic cell-mediated immunotherapy (Kudo-Saito et al., 2009). Zinc finger E-box-binding homeobox (ZEB1)-induced EMT results in relief of miR-200-mediated repression of programmed cell death 1 ligand (PD-L1) expression, a major inhibitory ligand for the programmed cell death protein (PD-1) immune-checkpoint protein on CD8+ cytotoxic T lymphocyte (CTL), subsequently the CD8+ T cell immunosuppression and metastasis (Chen et al., 2014). |
Known Modulating Factors
| Modulating Factor (MF) | Influence or Outcome | KER(s) involved |
|---|---|---|
Quantitative Understanding
Wnt signaling activates the CSCs to promote cancer malignancy (Reya & Clevers, 2005). The responses in KEs related to Wnt signaling, Frizzled activation, GSK3beta inactivation, beta-catenin activation, Snail, Zeb, and Twist activation are dose-dependently related. The quantification of EMT and cancer malignancy would require further investigation.
Considerations for Potential Applications of the AOP (optional)
AOP entitled “Increase in reactive oxygen species (ROS) and chronic ROS leading to human treatment-resistant gastric cancer” might be utilized for the development and risk assessment of anti-cancer drugs. EMT is involved in the acquisition of drug resistance, which is one of the critical features of cancer malignancy. The assessment of the activity of the EMT network would serve as a prediction of the adverse effects of or responsiveness to anti-cancer drugs (Tanabe et al., 2023). The detection methods for increases in ROS in this AOP have future regulatory potentials to assess the human health effects of radiation or ROS-related diseases. The detection methods for human treatment-resistant gastric cancer have future regulatory potentials to diagnose the diseases.
References
Gu, H., Huang, Y. Shen, Y. Liu, F. Zhou, Y. Jin, et al. (2018). Reactive Oxygen Species-Mediated Tumor Microenvironment Transformation: The Mechanism of Radioresistant Gastric Cancer. Oxid Med Cell Longev 2018 Vol. 2018 Pages 5801209. doi:10.1155/2018/5801209
Kudo-Saito, C., Shirako, H., Takeuchi, T., & Kawakami, Y. (2009). Cancer Metastasis Is Accelerated through Immunosuppression during Snail-Induced EMT of Cancer Cells. Cancer Cell, 15(3), 195-206. doi: 10.1016/j.ccr.2009.01.023
Nile, A. H., Mukund, S., Stanger, K., Wang, W., & Hannoush, R. N. (2017). Unsaturated fatty acyl recognition by Frizzled receptors mediates dimerization upon Wnt ligand binding. Proc Natl Acad Sci U S A, 114(16), 4147-4152. doi:10.1073/pnas.1618293114
Tanabe, S. (2018). Wnt Signaling and Epithelial-Mesenchymal Transition Network in Cancer. Research Journal of Oncology, 2(2), 3.
Tanabe, S., Quader, S., Cabral, H., & Ono, R. (2020a). Interplay of EMT and CSC in Cancer and the Potential Therapeutic Strategies. Front. Pharmacol. 11:904. doi: 10.3389/fphar.2020.00904
Tanabe, S., Quader, S., Ono, R., Cabral, H., Aoyagi, K., Hirose, A., Yokozaki, H., & Sasaki, H. (2020b). Molecular Network Profiling in Intestinal- and Diffuse-Type Gastric Cancer. Cancers, 12(12), 3833. https://doi.org/10.3390/cancers12123833
Tanabe, S., Quader, S., Ono, R., Cabral, H., Aoyagi, K., Hirose, A., Perkins, E.J., Yokozaki, H., & Sasaki H. (2023). Regulation of Epithelial–Mesenchymal Transition Pathway and Artificial Intelligence-Based Modeling for Pathway Activity Prediction. Onco, 3(1):13-25. doi: 10.3390/onco3010002