
This AOP is licensed under the BY-SA license. This license allows reusers to distribute, remix, adapt, and build upon the material in any medium or format, so long as attribution is given to the creator. The license allows for commercial use. If you remix, adapt, or build upon the material, you must license the modified material under identical terms.
AOP: 307
Title
Decreased testosterone synthesis leading to short anogenital distance (AGD) in male (mammalian) offspring
Short name
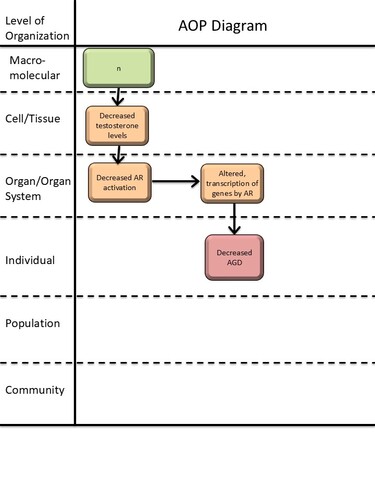
Graphical Representation
Point of Contact
Contributors
- Terje Svingen
Coaches
- Judy Choi
- Shihori Tanabe
OECD Information Table
| OECD Project # | OECD Status | Reviewer's Reports | Journal-format Article | OECD iLibrary Published Version |
|---|---|---|---|---|
| 1.90 | Under Review | Scientific Review | AOP report: adverse outcome pathway network for developmental androgen signaling inhibition leading to short anogenital distance in male offspring |
This AOP was last modified on February 04, 2026 16:08
Revision dates for related pages
| Page | Revision Date/Time |
|---|---|
| Decrease, circulating testosterone levels | January 27, 2025 03:37 |
| Decrease, androgen receptor activation | February 04, 2026 16:01 |
| Altered, Transcription of genes by the androgen receptor | April 05, 2024 09:28 |
| anogenital distance (AGD), decreased | December 22, 2022 05:18 |
| Decrease, intratesticular testosterone levels | January 27, 2025 11:33 |
| Altered, Transcription of genes by the AR leads to AGD, decreased | February 04, 2026 16:15 |
| Decrease, intratesticular testosterone leads to Decrease, circulating testosterone levels | May 07, 2025 04:19 |
| Decrease, AR activation leads to AGD, decreased | February 04, 2026 09:11 |
| Decrease, circulating testosterone levels leads to Decrease, AR activation | February 04, 2026 16:03 |
| Decrease, AR activation leads to Altered, Transcription of genes by the AR | April 05, 2024 08:50 |
| Decrease, intratesticular testosterone leads to AGD, decreased | May 06, 2025 07:11 |
| Decrease, circulating testosterone levels leads to AGD, decreased | May 06, 2025 07:03 |
| Dibutyl phthalate | November 29, 2016 18:42 |
| Bis(2-ethylhexyl) phthalate | November 29, 2016 18:42 |
Abstract
This AOP links decreased intratesticular testosterone levels with short anogenital distance (AGD) in male offspring. It does not yet contain an MIE, as several upstream mechanisms can lead to ‘reduced testosterone’ synthesis in fetal testis, such as inhibiting key steroidogenic enzymes. Testosterone is synthesized from cholesterol through several enzymatic steps, including those catalyzed by CYP enzymes such as CYP11 and CYP17. Once synthesized, testosterone is released into circulation and transported to target tissues where it initiates masculinization by binding to and activating the androgen receptor (AR) in target cells. Notably, testosterone can be converted to DHT by 5α-reductase, with DHT being a more potent AR agonist than testosterone; this testosterone-to-DHT conversion is critical during development for differentiation of male traits, including masculinization of the developing fetus, including differentiation of the levator ani/bulbocavernosus (LABC) muscle complex (Davey and Grossmann, 2016; Keller et al, 1996; Robitaille and Langlois, 2020). The LABC complex fails to develop in the absence or insufficiency of androgen signaling, as for instance observed in female fetuses.
A short AGD around birth is a marker for undervirilization of male fetuses and is associated with male reproductive disorders, including reduced fertility in adulthood (Schwartz et al, 2019). Although a short AGD is not necessarily ‘adverse’ from a human health perspective, it is considered an ‘adverse outcome’ in OECD test guidelines; AGD measurements are mandatory in specific tests for developmental and reproductive toxicity in chemical risk assessment (TG 443, TG 421/422, TG 414), with measurement guidance provided in OECD guidance documents 43 (OECD, 2008) and 151 (OECD, 2013).
A central event in this pathway is the inhibition of testosterone synthesis in the fetal testes, leading to reduced circulating testosterone levels and decreased DHT convertion by 5α-reductase. Insufficient DHT fails to effectively activate AR in target tissues, including the developing perineal region, which leads to failure to properly masculinize the perineum/LABC complex and ultimately a short AGD.
AOP Development Strategy
Context
This AOP was developed as part of an AOP network for developmental androgen signalling-inhibition leading to short AGD in male offspring. The other AOPs in this network are AOP 305 (5α-reductase inhibition leading to short AGD) and 306 (AR antagonism leading to short AGD).
Androgen signaling is critical for male sex differentiation during fetal life, and suboptimal signaling during critical life stages leads to under-masculinized offspring. Androgens, primarily testosterone and DHT, exert their effects by binding to and activating the AR is target cells. Blocking the AR basically blocks androgen signaling and masculinization of tissues that otherwise should masculinize in male fetuses. One morphometric marker for reduced fetal androgen action is shorter AGD compared to control males.
Strategy
For the AOP network development, the OECD AOP Developer’s Handbook was followed alongside pragmatic approaches (Svingen et al., 2021). Key events (KEs) and key event relationships (KERs) based on canonical knowledge from the ‘upstream anti-androgenic network’ (Draskau et al., 2024) were treated less stringently, while new units adhered to more rigorous systematic literature retrieval methods. An exception was the new canonical KER-3348 linking KE-3398 (decreased intratesticular testosterone) with KE-1690 (decreased circulating testosterone), which was developed using reviews and selected primary articles as examples.
KER-2820, linking KE-1614 (decreased AR activation) with AO-1688 (decreased AGD), was developed using a systematic weight of evidence (WoE) approach (Holmer et al., 2024). From an initial 826 publications, 557 were retained, with 71 selected for data extraction (82 datasets). Ultimately, 25 reliable datasets from in vivo studies on five model compounds (flutamide, procymidone, vinclozolin, finasteride, di-2-ethylhexyl phthalate) provided strong empirical support.
KER-3449, linking KE 2298 (decreased intratesticular testosterone) with AO-1688 (decreased AGD), and KER-3349, linking KE-1690 (decreased circulating testosterone) with AO-1688 (decreased AGD) were developed systematically (WoE approach) in parallel using the same search string to retrieve all articles pertaining to reduced testosterone and AGD. From an initial 649 publications, 40 in vivo studies were selected for data extraction and reliability evaluation. Of these, 24 reliable data sets were retrieved for KER-3449, and 10 reliable data sets were retrieved for KER-3349, providing strong empirical support for both KERs.
Conversely, KER-2127, linking KE-286 (altered AR transcription) with AO-1688, was developed semi-systematically, yielding only two relevant studies due to limited transcriptional data from perineal tissue exposed to anti-androgenic chemicals.
The overall AOP assessments followed the AOP Developer’s Handbook guidelines.
Summary of the AOP
Events:
Molecular Initiating Events (MIE)
Key Events (KE)
Adverse Outcomes (AO)
| Type | Event ID | Title | Short name |
|---|
| KE | 1690 | Decrease, circulating testosterone levels | Decrease, circulating testosterone levels |
| KE | 1614 | Decrease, androgen receptor activation | Decrease, AR activation |
| KE | 286 | Altered, Transcription of genes by the androgen receptor | Altered, Transcription of genes by the AR |
| KE | 2298 | Decrease, intratesticular testosterone levels | Decrease, intratesticular testosterone |
| AO | 1688 | anogenital distance (AGD), decreased | AGD, decreased |
Relationships Between Two Key Events (Including MIEs and AOs)
| Title | Adjacency | Evidence | Quantitative Understanding |
|---|
| Decrease, intratesticular testosterone leads to Decrease, circulating testosterone levels | adjacent | High | Moderate |
| Decrease, circulating testosterone levels leads to Decrease, AR activation | adjacent | High | Moderate |
| Decrease, AR activation leads to Altered, Transcription of genes by the AR | adjacent | Moderate | Low |
| Altered, Transcription of genes by the AR leads to AGD, decreased | non-adjacent | Moderate | Low |
| Decrease, AR activation leads to AGD, decreased | non-adjacent | High | Moderate |
| Decrease, intratesticular testosterone leads to AGD, decreased | non-adjacent | Moderate | Moderate |
| Decrease, circulating testosterone levels leads to AGD, decreased | non-adjacent | High | Moderate |
Network View
Prototypical Stressors
Life Stage Applicability
| Life stage | Evidence |
|---|---|
| Foetal | High |
Taxonomic Applicability
Sex Applicability
| Sex | Evidence |
|---|---|
| Male | High |
Overall Assessment of the AOP
Domain of Applicability
The upstream part of the AOP, converging on KE-286 (altered transcription of genes by the AR), has a broad applicability domain. It is built primarily on mammalian data and includes all life stages, but only males due to the specification of intratesticular testosterone in KE-2298. It could be extended to cover non-mammalian vertebrates by adding additional relevant knowledge, as previously discussed (Draskau et al, 2024). The overall applicability domain is limited by AO-1688 (decreased AGD). The AGD is strongly influenced by androgen action during critical fetal stages in mammals, with evidence from humans (Murashima et al, 2015; Thankamony et al, 2016), and from numerous gestational exposure studies in rats and mice to anti-androgenic chemicals (Gray et al, 2001; Schwartz et al, 2019). The male masculinization programming window occurs at a developmental stage included in the applicability domain of these AOPs and corresponds to around gestational day 16-20 in rats and gestation weeks 8-14 in humans (Welsh et al, 2008). Only males are included in the applicability domain since the male AGD, but not the female AGD, is shortened by decreased androgen action (Schwartz et al, 2019).
Essentiality of the Key Events
The essentiality of each key event (KE) was evaluated, meaning that if an upstream KE is blocked or does not occur, subsequent downstream KEs or the adverse outcome (AO) are prevented or altered. Both direct and indirect evidence of essentiality were assessed according to the OECD developer’s handbook (see Supplementary Table S1, 5yrajtnxaq_Supplementary_Table_S1_Essentiality_table_AOPs_305_307_forwiki_R2.pdf), with a summary provided in Table 1.
Table 1: Essentiality assessment of KEs of AOP 307.
|
Event |
Direct evidence |
Indirect evidence |
Contradictory evidence |
Overall essentiality assessment |
| KE-2298 | *** | Moderate | ||
|
KE-1690 |
|
*** |
|
Moderate |
|
KE-1614 |
*** |
*** |
|
High |
|
KE-286 |
|
*** |
|
Moderate |
*Low level of evidence (some support for essentiality), ** Intermediate level of evidence (evidence for impact on one or more downstream KEs), ***High level of evidence (evidence for impact on AO).
Evidence Assessment
Evidence for anti-androgenicity, by antagonizing the AR, is strong. In this AOP, most KERs are considered highly biologically plausible with strong empirical evidence in support of this assessment, both from human data and animal studies. The overall evidence assessment scores for each KER are summarized in the Table below:
|
ID |
Assessment score |
Rationale |
| KER-3448 | High | It is considered canonical knowledge that testis is primary site of testosterone synthesis, and that circulating T will be directly impacted by testis production. |
|
KER-2131 |
High |
It is well established that testosterone activates the AR and that decreased testosterone levels leads to decreased AR activation. |
|
KER-2124 |
High |
It is well established that the AR regulates gene transcription, and that decreased AR activity leads to altered gene transcription. |
| KER-3449 | High | It is well established that testis is the main site of testosterone synthesis and impacts circulating T levels, which again impacts masculinization, including AGD. |
| KER-3449 | High | It is well established that decreased serum testosterone levels impact masculinization of the male fetus, including a feminized AGD. |
|
KER-2820 |
High |
It is well established that decreased AR activity leads to decreased AGD in male offspring. |
|
KER-2127 |
Moderate |
It is highly plausible that altered gene transcription in the perineum leads to decreased AGD in male offspring. |
Known Modulating Factors
| Modulating Factor (MF) | Influence or Outcome | KER(s) involved |
|---|---|---|
| Genotype | Decreased AR activation with increased number of CAG repeats in the first axon of the AR (Chamberlain et al, 1994; Simanainen et al, 2011; Tut et al, 1997). | Identified in KER-2131, KER-2124 and KER-2820. |
Quantitative Understanding
The quantitative understanding between in vitro test data and in vivo is low. There is some quantitative understanding about the magnitude of reduction in explanted fetal testis testosterone production and effect on AGD (and other masculinization parameters) in rats, related to phthalate exposures. The dose-response relationship appears non-linear, with a low incidence rate of male under-virilization effects when testosterone production is reduced by less than 60% but with a steep increase in rate of malformations, including a decreasing length of the perineum, when testosterone is reduced by more than 60% (Earl Gray et al, 2024). This relationship has not been systematically evaluated for other chemicals.
Considerations for Potential Applications of the AOP (optional)
References
Bhasin S, Cunningham GR, Hayes FJ, Matsumoto AM, Snyder PJ, Swerdloff RS, Montori VM; Task Force, Endocrine Society (2010). Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 95(6):2536-59.
Chamberlain NL, Driver ED, Miesfeld RL (1994). The length and location of CAG trinucleotide repeats in the androgen receptor N-terminal domain affect transactivation function. Nucleic Acids Res 22(15):3181-6.
Davey RA, Grossmann M (2016). Androgen Receptor Structure, Function and Biology: From Bench to Bedside. Clin Biochem Rev 37(1):3-15.
Draskau MK, Rosenmai AK, Bouftas N, Johansson HKL, Panagiotou EM, Holmer ML, Elmelund E, Zilliacus J, Beronius A, Damdimopolou P, van Duursen M, Svingen T (2024). AOP Report: An Upstream Network for Reduced Androgen Signaling Leading to Altered Gene Expression of Androgen Receptor-Responsive Genes in Target Tissues. Environ Toxicol Chem 43(11):2329-2337.
Earl Gray L Jr (2023). Biologically relevant reductions in fetal testosterone and Insl3 induced by in utero exposure to high levels of di-isononyl phthalate (DINP) in male rats. Toxicol Appl Pharmacol 465:116454.
Earl Gray L Jr, Lambright CS, Evans N, Ford J, Conley JM (2024). Using targeted fetal rat testis genomic and endocrine alterations to predict the effects of a phthalate mixture on the male reproductive tract. Curr Res Toxicol. 7:100180. doi: 10.1016/j.crtox.2024.100180
Gray LE, Ostby J, Furr J, Wolf CJ, Lambright C, Parks L, Veeramachaneni DN, Wilson V, Price M, Hotchkiss A, Orlando E, Guillette L (2001). Effects of environmental antiandrogens on reproductive development in experimental animals. Hum Reprod Update 7(3):248-64.
Holmer ML, Zilliacus J, Draskau MK, Hlisníková H, Beronius A, Svingen T (2024). Methodology for developing data-rich Key Event Relationships for Adverse Outcome Pathways exemplified by linking decreased androgen receptor activity with decreased anogenital distance. Reprod Toxicol 128:108662.
Keller ET, Ershler WB, Chang C (1996). The androgen receptor: a mediator of diverse responses. Front Biosci 1:d59-71.
Murashima A, Kishigami S, Thomson A, Yamada G (2015). Androgens and mammalian male reproductive tract development. Biochim Biophys Acta 1849(2):163-70.
OECD (2008), Guidance Document on Mammalian Reproductive Toxicity Testing and Assessment, OECD Series on Testing and Assessment, No. 43, OECD Publishing, Paris.
OECD (2013) Guidance document in support of the test guideline on the extended one generation reproductive toxicity study no. 151.
Robitaille J, Langlois VS (2020). Consequences of steroid-5α-reductase deficiency and inhibition in vertebrates. Gen Comp Endocrinol 290:113400.
Schwartz CL, Christiansen S, Vinggaard AM, Axelstad M, Hass U, Svingen T (2019). Anogenital distance as a toxicological or clinical marker for fetal androgen action and risk for reproductive disorders. Arch Toxicol 93(2):253-272.
Simanainen U, Brogley M, Gao YR, Jimenez M, Harwood DT, Handelsman DJ, Robins DM (2011). Length of the human androgen receptor glutamine tract determines androgen sensitivity in vivo. Mol Cell Endocrinol. 6;342(1-2):81-6. doi: 10.1016/j.mce.2011.05.011.
Supakar PC, Song CS, Jung MH, Slomczynska MA, Kim JM, Vellanoweth RL, Chatterjee B, Roy AK (1993). A novel regulatory element associated with age-dependent expression of the rat androgen receptor gene. J Biol Chem 268(35):26400-8.
Svingen T, Villeneuve DL, Knapen D, Panagiotou EM, Draskau MK, Damdimopoulou P, O'Brien JM (2021). A Pragmatic Approach to Adverse Outcome Pathway Development and Evaluation. Toxicol Sci 184(2):183-190.
Thankamony A, Pasterski V, Ong KK, Acerini CL, Hughes IA (2016). Anogenital distance as a marker of androgen exposure in humans. Andrology 4(4):616-25.
Tut TG, Ghadessy FJ, Trifiro MA, Pinsky L, Yong EL (1997). Long polyglutamine tracts in the androgen receptor are associated with reduced trans-activation, impaired sperm production, and male infertility. J Clin Endocrinol Metab 82(11):3777-82.
Welsh M, Saunders PT, Fisken M, Scott HM, Hutchison GR, Smith LB, Sharpe RM (2008). Identification in rats of a programming window for reproductive tract masculinization, disruption of which leads to hypospadias and cryptorchidism. J Clin Invest 118(4):1479-90.
Wu D, Lin G, Gore AC (2009). Age-related changes in hypothalamic androgen receptor and estrogen receptor alpha in male rats. J Comp Neurol 512(5):688-701.