
This AOP is licensed under the BY-SA license. This license allows reusers to distribute, remix, adapt, and build upon the material in any medium or format, so long as attribution is given to the creator. The license allows for commercial use. If you remix, adapt, or build upon the material, you must license the modified material under identical terms.
AOP: 48
Title
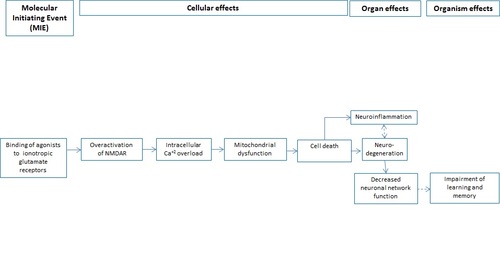
Binding of agonists to ionotropic glutamate receptors in adult brain causes excitotoxicity that mediates neuronal cell death, contributing to learning and memory impairment.
Short name
Graphical Representation
Point of Contact
Contributors
- Anna Price
Coaches
OECD Information Table
| OECD Project # | OECD Status | Reviewer's Reports | Journal-format Article | OECD iLibrary Published Version |
|---|---|---|---|---|
| 1.23 | WPHA/WNT Endorsed | iLibrary link |
This AOP was last modified on April 29, 2023 16:02
Revision dates for related pages
| Page | Revision Date/Time |
|---|---|
| Increase, Mitochondrial dysfunction | February 11, 2026 07:06 |
| Impairment, Learning and memory | July 26, 2024 09:54 |
| Increase, Cell injury/death | May 27, 2024 07:23 |
| N/A, Neurodegeneration | February 23, 2021 05:07 |
| Overactivation, NMDARs | July 14, 2024 11:45 |
| Increased, Intracellular Calcium overload | June 26, 2020 04:45 |
| Decreased, Neuronal network function in adult brain | September 16, 2017 10:15 |
| Binding of agonist, Ionotropic glutamate receptors | September 16, 2017 10:15 |
| Neuroinflammation | July 15, 2022 09:54 |
| Overactivation, NMDARs leads to Increased, Intracellular Calcium overload | September 10, 2023 20:11 |
| Cell injury/death leads to N/A, Neurodegeneration | September 10, 2023 19:25 |
| Neuroinflammation leads to N/A, Neurodegeneration | February 23, 2021 05:47 |
| N/A, Neurodegeneration leads to Neuroinflammation | June 13, 2018 09:35 |
| Binding of agonist, Ionotropic glutamate receptors leads to Overactivation, NMDARs | November 29, 2016 20:44 |
| Increased, Intracellular Calcium overload leads to Increase, Mitochondrial dysfunction | November 29, 2016 20:08 |
| Increase, Mitochondrial dysfunction leads to Cell injury/death | November 29, 2016 20:08 |
| Cell injury/death leads to Neuroinflammation | July 15, 2022 08:26 |
| Decreased, Neuronal network function in adult brain leads to Impairment, Learning and memory | November 29, 2016 20:23 |
| N/A, Neurodegeneration leads to Decreased, Neuronal network function in adult brain | November 29, 2016 20:24 |
Abstract
Under physiological conditions activation of glutamate ionotropic receptors such as N-methyl-D-aspartate (NMDARs), alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionate (AMPARs) and kainate (KARs) is responsible for basal excitatory synaptic transmission and main forms of synaptic plasticity such as long-term potentiation (LTP) and long-term depression (LTD) that are fundamental for learning and memory processes (Schrattenholz and Soskic, 2006). However, sustained (direct or indirect) over-activation of these receptors can induce excitotoxic neuronal cell death. Indeed, mainly increased Ca2+ influx through NMDARs promotes many pathways of toxicity due to generation of free radical species, reduced ATP production, endoplasmic reticulum (ER) stress and protein aggregation. Neuronal injury induced by over-activation of these receptors and the excessive Ca2+ influx is considered an early key event of excitotoxicity. Additionally, the excessive activation of NMDARs has been found to play a significant role in a variety of neurological disorders ranging from acute hypoxic-ischemic brain injury (Barenger et al., 2001) to chronic neurodegenerative diseases (Mehta et al., 2013). The proposed AOP is relevant to adult neurotoxicity testing. A molecular initiating event (MIE) has been defined as a direct binding of agonists to NMDARs or indirect, through prior activation of AMPARs and/or KARs resulting in sustained NMDARs over-activation causing excitotoxic neuronal cell death, mainly in hippocampus and cortex, two brain structures fundamental for learning and memory processes. The AOP is based on the empirical support describing (1) domoic acid (DomA) induced excitotoxicity triggered by indirect (through KARs/AMPARs) NMDARs over-activation leading to impaired learning and memory and (2) glufosinate (GLF) induced excitotoxicity that through direct binding to NMDARs causes convulsions and memory loss (Lanz et al., 2014). GLF is the methylphosphine analog of L-glutamate, used as a component of bactericidal and fungicidal herbicidal. DomA, a natural toxin that accumulates in mussels and shellfish is also an analogue of L-glutamate and among the most prominent features described after human exposure to DomA is memory impairment (Lefebvre and Robertson, 2010). DomA and GLF are described as the examples of the stressors due to large amounts of published data (especially in the case of DomA), however this AOP is relevant to any agonist that directly or indirectly cause NMDARs over-activation. Some of the known agonists selective for the NMDARs are derived from the naturally occurring amino acids such as ibotenic acid, homocysteine and l-aspartate and polyamines like spermidine.
AOP Development Strategy
Context
Strategy
Summary of the AOP
Events:
Molecular Initiating Events (MIE)
Key Events (KE)
Adverse Outcomes (AO)
| Type | Event ID | Title | Short name |
|---|
| MIE | 875 | Binding of agonist, Ionotropic glutamate receptors | Binding of agonist, Ionotropic glutamate receptors |
| KE | 177 | Increase, Mitochondrial dysfunction | Increase, Mitochondrial dysfunction |
| KE | 55 | Increase, Cell injury/death | Cell injury/death |
| KE | 352 | N/A, Neurodegeneration | N/A, Neurodegeneration |
| KE | 388 | Overactivation, NMDARs | Overactivation, NMDARs |
| KE | 389 | Increased, Intracellular Calcium overload | Increased, Intracellular Calcium overload |
| KE | 618 | Decreased, Neuronal network function in adult brain | Decreased, Neuronal network function in adult brain |
| KE | 188 | Neuroinflammation | Neuroinflammation |
| AO | 341 | Impairment, Learning and memory | Impairment, Learning and memory |
Relationships Between Two Key Events (Including MIEs and AOs)
| Title | Adjacency | Evidence | Quantitative Understanding |
|---|
| Overactivation, NMDARs leads to Increased, Intracellular Calcium overload | adjacent | Moderate | |
| Cell injury/death leads to N/A, Neurodegeneration | adjacent | Moderate | |
| Neuroinflammation leads to N/A, Neurodegeneration | adjacent | Moderate | |
| N/A, Neurodegeneration leads to Neuroinflammation | adjacent | Moderate | |
| Binding of agonist, Ionotropic glutamate receptors leads to Overactivation, NMDARs | adjacent | High | |
| Increased, Intracellular Calcium overload leads to Increase, Mitochondrial dysfunction | adjacent | High | Moderate |
| Increase, Mitochondrial dysfunction leads to Cell injury/death | adjacent | Moderate | Low |
| Cell injury/death leads to Neuroinflammation | adjacent | Low | Low |
| Decreased, Neuronal network function in adult brain leads to Impairment, Learning and memory | adjacent | Moderate | Low |
| N/A, Neurodegeneration leads to Decreased, Neuronal network function in adult brain | adjacent | Low |
Network View
Prototypical Stressors
Life Stage Applicability
| Life stage | Evidence |
|---|---|
| Adults | High |
Taxonomic Applicability
Sex Applicability
| Sex | Evidence |
|---|---|
| Male | High |
| Female | High |
Overall Assessment of the AOP
The aim of the present AOP is to construct a linear pathway that captures the KEs and KERs that occur after binding of agonist to NMDA receptor in hippocampal and cortical neurons of adults. The majority of the KEs of the AOP are characterised by MODERATE essentiality for the AO (loss or reduction of cognitive function )or other KEs that follow. The biological plausibility in the majority of KERs is rated STRONG as there is extensive mechanistic understanding. However, the empirical support for the majority of presented KERs cannot be rated high as in most occasions the KEup and KEdown of a KER has not been investigated simultaneously, under the same experimental protocol or not in the suggested brain regions (cortex and hippocampus).
Domain of Applicability
Life Stage Applicability: This AOP is applicable for adults. However, studies exploring the neurotoxic effects of DomA on the developing nervous system demonstrate that DomA can cause neurobehavioral, biochemical and morphological effects similar to the ones observed in adult animals (reviewed in Costa et al., 2010). The DomA doses required to cause these effects in developing organisms are one to two orders of magnitude lower than those needed for loss or reduction of cognitive function in adults. This difference has been attributed to toxicokinetic and/or toxicodynamic particularities that exist between adults and children.
Taxonomic Applicability: The data used to support the KERs in this AOP derives from experimental studies conducted in primates, rats and mice or cell cultures of similar origin as well as from human epidemiological studies or clinical cases of DomA poisoning. The majority of the KEs in this AOP seem to be highly conserved across species. It remains to be proved if these KERs of the present AOP are also applicable for other species rather than human, primates, rats or mice. Increasing evidence from sea lions exposed to DomA further supports some of the KERs of the present AOP.
Sex Applicability: The majority of the studies addressing the KEs and KERs of this AOP have been carried out mainly in male laboratory animals. Few studies are available in females and some of them compare the effects between females and males. It appears that this AOP is applicable for both females and males.
Essentiality of the Key Events
1) Essentiality of KE "NMDARs, Overactivation" for the KE "Cell death" is MODERATE. NMDARs play a central role in excitotoxic neuronal injury. Over-activation of these receptors causes disruption of Ca2+ homeostasis that through mitochondrial dysfunction triggers signals leading to apoptotic or necrotic death. However, the ways that cells respond to mitochondrial injury vary and often are considered unclear and controversial (Pivovarova and Andrews, 2010). However, NMDAR antagonists failed to reverse these Ca2+ induced cell deaths, leading to suggestions that NMDAR-independent pathways that involve α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPARs), acid-sensing channels and transient receptor potential channels might be also responsible for excitotoxic neuronal injury (Pivovarova and Andrews, 2010). Several agonists have higher affinity than NMDA itself but are not relevant for behavioural studies as NMDA activation leads to epilepsy and cell death, a common approach to induce neurotoxic lesions.
2) Essentiality of KE "Calcium influx, Increased" for the KE "Cell death" is MODERATE. Ca2+ plays important role in excitotoxicity but the mechanisms involved in excitotoxic cell death are still debated (Berliocchi et al., 2005). Depending on the extent and the duration of the Ca2+ influx, neurons survive, die through apoptotic mechanisms in case of sustained slow Ca2+ influx, or undergo necrosis when rapid high Ca2+ influx occurs. Over-expression of the endogenous calpain inhibitor, calpastatin, or the calpain-resistant isoform the Na+/Ca2+ exchanger 2 (NCX2) prevents Ca2+ overload and protects neurons from excitotoxicity (Bano et al., 2005).
3) Essentiality of KE "Mitochondria dysfunction" for the AO "Impairment of learning and memory" is STRONG. ROS is known to have a negative effect on synaptic plasticity and learning and memory (reviewed in Lynch, 2004). H2O2 inhibits LTP both in vitro and in vivo, which is associated with increased ROS. A negative correlation has been found between ROS concentration in hippocampus and ability of rats to sustain LTP. Administration of antioxidants, vitamins E and C, reverses the inhibitory effects of stress on LTP and prevents the increase of ROS in hippocampus. In transgenic mice that overexpress superoxide dismutase (SOD), the enzyme which catalyzes the conversion of superoxide to H2O2, the LTP in CA1 is inhibited. Intracerebroventricular injection of H2O2, at a concentration which increases ROS levels in hippocampus, impairs LTP that is prevented after pretreatment with the antioxidant phenylarsine oxide. Knocking down Forkhead box protein O1 (FoxO1) in mice, which is an important regulator of mitochondrial function, reverses mitochondrial abnormalities and cognitive impairment induced by DA in mice (Wu et al., 2013).
4) Essentiality of KE "Mitochondria dysfunction" for the KE "Cell death" is MODERATE. There is a considerable number of mitochondrial associated processes that lead to necrotic or apoptotic cell death such as uncoupling of oxidative phosphorylation, activation of the mitochondrial permeability transition pore (MPTP), release of pro-apoptotic proteins, activation of poly(ADP-ribose) polymerase-1 and proteases such as calpain, increased levels of and delayed Ca2+ de-regulation (Pivovarova and Andrews, 2010). Although the understanding of these mechanisms is clearly established, the cascade of events and the significance of them are less clear (Pivovarova and Andrews, 2010). A significant body of evidence, both clinical and experimental, supports a role for the mitochondrial permeability transition pore in excitotoxicity (reviewed in Pivovarova and Andrews, 2010). However, the effects of cyclosporin A, the classical MPTP inhibitor, on neuronal mitochondria are inconsistent raising doubts about its role in neural cell death. However, ADP/ATP translocator deficiency, which is not essential for MPTP but does regulate pore opening, protects neurons against excitotoxicity. Furthermore, MPTP opening renders neurons vulnerable to excitotoxicity.
Evidence Assessment
The table provides a summary of the biological plausibility and the empirical support for each KER described in this AOP based on "Annex 1: Guidance for assessing relative level of confidence in the overall AOP based on rank ordered elements" found in the User's Handbook.
More information about the evidence that support these KERs and the relevant literature can be found in each KER description.
The main base for the overall scoring is that the empirical support coming from the experiments with one stressor (domoic acid, DomA). However this AOP is not specific for DomA, it is applicable to any chemicals that act as NMDARs agonists.
| KERs WoE | Biological plausibility | Does KEup occurs at lower doses than KEdown? | Does KEup occurs at earlier time points than KE down? | Is there higher incidence of KEup than of KEdown? | Inconsistencies/Uncertainties |
| Binding of agonist to NMDARs directly leads to NMDARs overactivation | Extensive understanding | N/A | Yes | N/A | Limited conficting data |
| NMDARs overactivation directly leads to increased calcium influx | Extensive understanding | Same dose | Yes | Not investigated | Limited conficting data |
| Increased calcium influx indirectly leads to mitochondrial dysfunction | Extensive understanding | Same dose | Yes | Yes | No conflicting data |
| Mitochondrial dysfunction directly leads to cell death | Extensive understanding | Same dose | Yes | Yes | Limited conficting data |
| Cell death leads to Neurodegeneration | Extensive understanding | Same dose | Yes | Yes | Limited conficting data |
| Cell death leads to Neuroinflammation | Extensive understanding | Not investigated | Not investigated | Not investigated | N/A |
| Neurodegeneration directly leads to Decreased neuronal network function | Extensive understanding | Not investigated | Not investigated | Not investigated | N/A |
| Decreased neuronal network function indirectly leads to loss or reduction of cognitive function | Scientific understanding is not completely established | Not investigated | Not investigated | Not investigated | N/A |
Known Modulating Factors
Quantitative Understanding
Considerations for Potential Applications of the AOP (optional)
Exposure to xenobiotics can potentially affect the nervous system resulting in neurobehavioral alterations and/or neurological clinical symptoms. To assess the neurotoxic properties of compounds, current testing largely relies on neurobehavioural tests in laboratory animals, histopathological analysis, neurochemical and occasionally electrophysiological observations. Throughout the years, a significant number of methods have been developed to assess neurobehaviour in laboratory animals and a comprehensive summary of them can be found in OECD Series on testing and assessment, number 20, Guidance Document for Neurotoxicity Testing (2004). This document is considered an essential supplement to a substantial number of already existing OECD Test Guidelines that are applied to gain information on the neurotoxicity properties of chemical compounds. Namely, these are: general Test Guidelines such as single dose toxicity (e.g. OECD 402, 403, 420, 423 and 425), repeated dose toxicity (e.g. OECD 407 and 408), chronic exposure (e.g. OECD 452) as well as Test Guidelines specifically developed for the study of neurotoxicity in adult laboratory animals, such as OECD Test Guideline for Neurotoxicity (424).
Learning and memory is an important endpoint and a wide variety of tests to assess chemical effects on cognitive functions is available and used for the study of neurotoxicity. Some of these tests that allow the appreciation of cognitive function in laboratory animals are: habituation, ethologically based anxiety tests (elevated plus maze test, black and white box test, social interaction test), conditioned taste aversion (CTA), active avoidance, passive avoidance, spatial mazes (Morris water maze, Biel water maze, T-maze), conditional discrimination (simple discrimination, matching to sample), delayed discrimination (delayed matching-to-sample, delayed alternation) and eye-blink conditioning.
The present AOP can potentially provide the basis for development of a mechanistically informed IATA for neurotoxicity. The construction of IATA for predicting neurotoxic effects in adults is expected to make use of more than one AOP within an interconnected network in order to take into consideration all critical biological processes that may contribute to impairment of learning and memory in adults. Through this network, identification of KEs and KERs common across multiple AOPs can emerge that should be considered during IATA construction and that may inform also in vitro assay development. The development of alternative assays would allow screening of chemicals for potential NMDAR activators and reducing the use of in vivo studies.
Results from assays based on the KEs of this AOP can serve to interpret and accept results that derive from non-standard test methods. Omics data from toxicogenomic, transcriptomic, proteomic, and metabolomic studies can be interpreted in a structured way using this AOP that is relevant to adult neurotoxicity. Currently learning and memory testing is not required by the OECD TG 424. This AOP could serve as a base for chemical evaluation with potential to cause impairment of learning and memory. The assay development would refer to the identified in this AOP KEs that could form a testing strategy for identifying chemicals with potential to cause cognitive deficit. Finally, this AOP could provide the opportunity to group chemicals using not only chemical properties but also mechanistic information that can later inform data gap filling by read-across and predict neurotoxic properties of a target substance.
References
Bano D, Young K.W, Guerin C.J, Lefeuvre R, Rothwell N.J, Naldini L, Rizzuto R, Carafoli E, Nicotera P. Cleavage of the plasma membrane Na+/Ca2+ exchanger in excitotoxicity. Cell, 2005, 120: 275-285.
Berliocchi L, Bano D, Nicotera P. Ca2+ signals and death programmes in neurons. Philos Trans R Soc Lond B Biol Sci., 2005, 360: 2255-2258.
Costa LG, Giordano G, Faustman EM. Domoic acid as a developmental neurotoxin. Neurotoxicology, 2010, 31(5):409-23.
Health Effects Test Guidelines OPPTS 870.6300 Developmental Neurotoxicity Study, US EPA, Prevention, Pesticides and Toxic Substances (7101), EPA 712-C-96, 239, 1996, 1-14.
Lynch MA. Long-term potentiation and memory. Physiol Rev. 2004, 84(1):87-136.
OECD (2004) Series on testing and assessment number 20, Guidance document for neurotoxicity testing.
OECD (2007). Test Guideline 426. OECD Guideline for Testing of Chemicals. Developmental Neurotoxicity Study. http://www.oecd.org/document/55/0,3343,en_2649_34377_2349687_1_1_ 1_1,00.html
OECD (2008) Nr 43 GUIDANCE DOCUMENT ON MAMMALIAN REPRODUCTIVE TOXICITY TESTING AND ASSESSMENT. ENV/JM/MONO(2008)16
Pivovarova NB, Andrews SB. Calcium-dependent mitochondrial function and dysfunction in neurons. FEBS J., 2010, 277: 3622-3636.
Wu DM, Lu J, Zhang YQ, Zheng YL, Hu B, Cheng W, Zhang ZF, Li MQ. Ursolic acid improves domoic acid-induced cognitive deficits in mice. Toxicol Appl Pharmacol., 2013, 271:127-36.