AOP ID and Title:
Graphical Representation
Status
| Author status | OECD status | OECD project | SAAOP status |
|---|---|---|---|
| Under development: Not open for comment. Do not cite |
Abstract
This AOP links inhibition of 5α-reductase during fetal life with increased nipple/areola retention (NR) in male rodent offspring. NR, measured around 2 weeks postpartum, is a marker for disrupted masculinization of male rodents (primarily investigated in laboratory rats and mice) and is associated with general feminization of male offspring.
5α-reductase is an enzyme that converts testosterone to dihydrotestosterone (DHT). In normal male reproductive development, DHT activates the androgen receptor (AR) in many peripheral reproductive tissues to drive differentiation of the male phenotype, including regression of nipple anlagen in male rats and mice. While testosterone also acts directly at the AR, DHT is 5-10 times more potent and in tissues peripheral to the testes, conversion to DHT is necessary for proper masculinization (Amato et al., 2022; Davey & Grossmann, 2016).
This AOP delineates the evidence that inhibition of 5α-reductase reduces DHT levels and consequently AR activation, causing retention of nipples in male rodents. The AOP is supported by in vitro experiments upstream of AR activation and by in vivo studies downstream of AR activation. Downstream of a reduction in AR activation, the molecular mechanisms of NR are unclear, highlighting a knowledge gap in this AOP and potential for further development.
The confidence in each of the KERs comprising the AOP is judged as high, with both high biological plausibility and high confidence in empirical evidence. The mechanistic link between KE-286 (‘altered, transcription of genes by AR’) and AO 1786 (‘increase, nipple retention’) is not established, but given the high confidence in the KERs, the overall confidence in the AOP is judged as high.
The AOP supports the regulatory application of NR as a measure of endocrine disruption relevant for human health and the use of NR as an indicator of anti-androgenicity in environmentally relevant species. Even though NR cannot be directly translated to a human endpoint, the AOP is considered human relevant since NR is a clear readout of reduced androgen action and masculinization during development and is considered an ‘adverse outcome’ in OECD test guidelines (TG 443, TG 421, TG 422). The AOP also holds utility for informing on anti-androgenicity more generally, as this modality is highly relevant across mammalian species and vertebrates more broadly due to the conserved nature of the AR and its implication in sexual differentiation across species.
Background
This AOP is a part of an AOP network for reduced AR activation leading to increased NR in male offspring. The other AOPs in this network are AOP 344 (‘Androgen receptor antagonism leading to increased nipple retention in male (rodent) offspring’), and AOP 575 (‘Decreased testosterone synthesis leading to increased nipple retention in male (rodent) offspring’). The purpose of the AOP network is to organize the well-established evidence for anti-androgenic mechanisms-of-action leading to increased NR. It can be used in identification and assessment of endocrine disruptors and to inform predictive toxicology, identification of knowledge gaps for investigation and method development.
This work received funding from the European Food and Safety Authority (EFSA) under Grant agreement no. GP/EFSA/PREV/2022/01.
Summary of the AOP
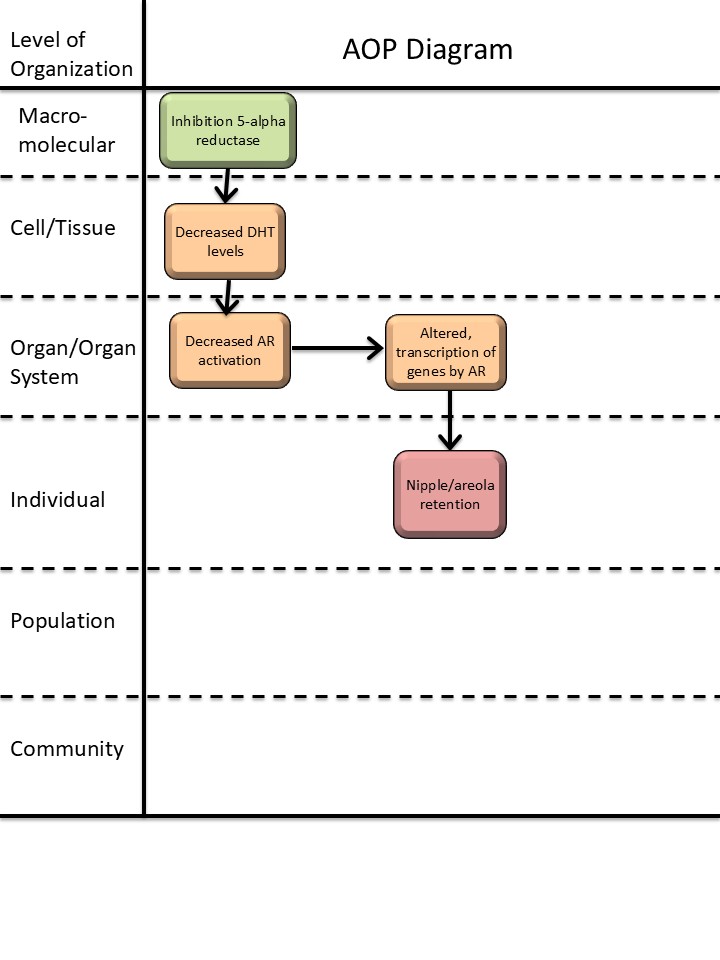
Events
Molecular Initiating Events (MIE), Key Events (KE), Adverse Outcomes (AO)
| Sequence | Type | Event ID | Title | Short name |
|---|---|---|---|---|
| MIE | 1617 | Inhibition, 5α-reductase | Inhibition, 5α-reductase | |
| KE | 1613 | Decrease, dihydrotestosterone (DHT) levels | Decrease, DHT level | |
| KE | 1614 | Decrease, androgen receptor activation | Decrease, AR activation | |
| KE | 286 | Altered, Transcription of genes by the androgen receptor | Altered, Transcription of genes by the AR | |
| AO | 1786 | Nipple retention (NR), increased | nipple retention, increased |
Key Event Relationships
| Upstream Event | Relationship Type | Downstream Event | Evidence | Quantitative Understanding |
|---|---|---|---|---|
| Inhibition, 5α-reductase | adjacent | Decrease, dihydrotestosterone (DHT) levels | High | |
| Decrease, dihydrotestosterone (DHT) levels | adjacent | Decrease, androgen receptor activation | High | |
| Decrease, androgen receptor activation | adjacent | Altered, Transcription of genes by the androgen receptor | High | |
| Decrease, androgen receptor activation | non-adjacent | Nipple retention (NR), increased | High |
Stressors
| Name | Evidence |
|---|---|
| Finasteride |
Overall Assessment of the AOP
Domain of Applicability
Life Stage Applicability| Life Stage | Evidence |
|---|---|
| Foetal | High |
| Sex | Evidence |
|---|---|
| Male | High |
The upstream part of the AOP has a broad applicability domain, but KER 3348 (Decrease, AR activation, leads to increased nipple retention) is considered only directly applicable to male rodent (current evidence primarily from rats and mice) during fetal life, restricting the applicability of the AOP. NR is specific to animals with sexual dimorphism in the number of nipples, a feature most prominently investigated in laboratory rats and mice. It is, however, biologically plausible that the AOP is applicable to other rodent species. The process of retention of nipples by disruption of androgen programming happens in the fetal life stage, but the AO is detected postnatally. In the males of mice and rats, the nipple anlagen are programmed during fetal development by androgens to regress, leading to no visible nipples in males postnatally, while females exhibit nipples. This AOP only contains empirical evidence for the applicability to male rats, but the AOP is considered equally applicable to male mice, as these also normally exhibit nipple regression stimulated by androgens. Moreover, the AOP is relevant for other taxa, including humans, as NR in male rodents indicates a reduction in fetal masculinization. NR is therefore included as a mandatory endpoint in multiple OECD Test Guideline studies for developmental and reproductive toxicity and is considered applicable as an adverse outcome to set NOAELs and LOAELs of substances in human health risk assessments.
Essentiality of the Key Events
|
Event |
Evidence |
Uncertainties, inconsistencies and contradictory evidence |
|
MIE-1617 Inhibition, 5α-reductase
HIGH: This MIE is usually measured in vitro, whereas the downstream events in the AOP are usually measured in vivo. Canonical knowledge of normal male reproductive development provides strong support for essentiality, along with 5α-reductase knockout models and models using exposure to 5α-reductase inhibitors. |
Biological plausibility provides strong support for the essentiality of this event, as DHT, produced by 5α-reductase, is a ligand of the AR and a primary driver of normal regression of nipple anlagen in male fetuses (Imperato-McGinley et al., 1986).
Indirect evidence of impact of inhibition of 5α-reductase (MIE-1617) in vitro on AR activity in vitro: • Finasteride, a specific inhibitor of 5α-reductase, can decrease proliferation of prostate cancer cells in vitro, a proxy read-out of AR activity (Bologna et al., 1995).
Direct evidence of impact of inhibition of 5α-reductase (MIE-1617) in vivo on decreased DHT levels (KE-1613): • Lack of 5α-reductase type 2 activity by e.g. inhibitor or KO decrease DHT levels locally in tissues and blood. This is demonstrated in humans, rats, monkeys, and mice (Robitaille & Langlois, 2020).
Indirect evidence of impact of inhibition of 5α-reductase (MIE-1617) in vivo on decreased DHT levels (KE-1613): • Men with androgenic alopecia treated with finasteride or dutasteride presented with decreased DHT levels in serum (Clark et al., 2004; Drake et al., 1999).
Direct evidence of impact of inhibition of 5α-reductase (MIE-1617) in vivo on increased nipple retention (AO-1786): • Exposure to the 5α-reductase inhibitors leads to increased retention of nipples in male offspring after in utero exposure (Christiansen et al., 2009; Imperato-McGinley et al., 1986). |
|
|
KE-1613 Decreased, DHT levels
HIGH: Canonical knowledge of normal male reproductive development provides strong support for essentiality, along with rescue studies specifically demonstrating how DHT is essential for normal regression of nipple anlagen in male offspring.
|
Biological plausibility provides strong support for the essentiality of this event. Androgens are AR ligands and main drivers for the regression of nipple anlagen in male offspring (Goldman et al., 1976), with DHT playing an important role (Imperato-McGinley et al., 1986).
Indirect evidence of impact of decreased DHT levels (KE-1613) on AR activity in vivo (KE-1614): • Androgen deprivation is used as treatment for prostate cancer, including 5α-reductase inhibitors, to reduce DHT levels and cancer growth (Aggarwal et al., 2010).
Indirect evidence of impact of decreased DHT levels (KE-1613) on AR activity in vitro: • Increasing concentrations of DHT lead to increasing AR activation in vitro in AR reporter gene assays (OECD, 2023; Williams et al., 2017).
Indirect evidence of impact of decreased DHT levels (KE-1613) on AR activity in vivo: • 5α-reductase 2 deficiency is an autosomal recessive condition in which 46,XY subjects with bilateral testes and normal testosterone production have impaired virilization during fetal life due to diminished DHT (Mendonca et al., 2016).
Direct evidence of impact of decreased DHT levels (KE-1613) on increased nipple retention (AO-1786). • Nipple formation is inhibited in female rat fetuses exposed to DHT during gestation (Goldman et al., 1976). • Exposure to the 5α-reductase inhibitor 390 MSD leads to increased retention of nipples in male rats after in utero exposure, whereas simultaneous exposure to DHT reverses the effects (Imperato-McGinley et al., 1986). |
|
|
KE-1614 Decreased, AR activation
HIGH: There is experimental evidence from mutant mice insensitive to androgens showing that the AR is essential for nipple retention in male offspring. There is also evidence from exposure studies in animals that substances antagonizing AR induce nipple retention in male pups. |
Biological plausibility provides strong support for the essentiality of this event, as AR activation is critical for normal regression of nipple anlagen in male embryos.
Indirect evidence of the impact of decreased AR activation (KE-1614) on altered gene transcription by AR (KE-286): • Exposure to known anti-androgenic chemicals induces a changed gene expression pattern, e.g. in neonatal pig ovaries (Knapczyk-Stwora et al., 2019).
Direct evidence of the impact of decreased AR activation (KE-1614) on altered gene transcription by AR (KE-286): • Male AR KO mice have altered gene expression pattern in a broad range of organs (see KER-2124).
Indirect evidence of impact of decreased AR activation (KE-1614) on increased nipple retention (AO-1786): • Rat in vivo exposure to vinclozolin, procymidone and flutamide, which are known AR antagonists, leads to increased nipple retention in offspring (see KER-3348).
Direct evidence of impact of decreased AR activation (KE-1614) on increased nipple retention (AO-1786): • Male Tfm mutant mice, which are insensitive to androgens and believed to be so due to a nonfunctional androgen receptor, present with retained nipples (Kratochwil & Schwartz, 1976) |
|
|
KE-286 Altered, trans. of genes by AR
LOW: Strongest support for essentiality comes from biological plausibility. However, exact transcriptional effects and causality remain to be fully characterized. |
Biological plausibility provides support for the essentiality of this event. AR is a nuclear receptor and transcription factor regulating transcription of genes, and androgens, acting through AR, are essential for normal regression of nipple anlagen in male fetuses. |
There are currently no AR-responsive genes proved to be causally involved in nipple retention, and it is known that AR can also signal through non-genomic actions (Leung & Sadar, 2017). |
|
Event |
Direct evidence |
Indirect evidence |
Contradictory evidence |
Overall essentiality assessment |
|
MIE-1617 |
*** |
** |
|
High |
|
KE-1613 |
*** |
** |
|
High |
|
KE-1614 |
*** |
*** |
|
High |
|
KE-286 |
|
|
|
Low (biological plausibility) |
*Low level of evidence (some support for essentiality), ** Intermediate level of evidence (evidence for impact on one or more downstream KEs), ***High level of evidence (evidence for impact on AO).
Weight of Evidence Summary
The confidence in each of the KERs comprising the AOP is judged as high, with both high biological plausibility and high confidence in empirical evidence. The mechanistic link between KE-286 (‘altered, transcription of genes by AR’) and AO 1786 (‘increase, nipple retention’) is not established, but given the high confidence in the KERs, the overall confidence in the AOP is judged as high.
|
KER |
Biological Plausibility |
Empirical Evidence |
Rationale |
|
KER-1880 Inhibition, 5α-reductase leads to a decrease, DHT levels |
High |
High (canonical) |
It is well established that 5α-reductase converts testosterone to DHT. In vitro, in vivo and human studies with 5α-reductase inhibitors have shown that the stressors dose-dependently decrease formation of DHT. |
|
KER-1935 Decrease, DHT levels leads to a decrease, AR activation |
High |
High (canonical) |
It is well established that DHT activates the AR. Direct evidence for this KER is not possible since KE 1614 can currently not be measured and is considered an in vivo effect. Indirect evidence using proxy read-outs of AR activation, either in vitro or in vivo, strongly supports the relationship. |
|
KER-2124 Decrease, AR activation leads to altered transcription of genes by AR |
High |
High (canonical) |
It is well established that the AR regulates gene transcription. In vivo animal studies and human genomic profiling show tissue-specific changes to gene expression upon disruption of AR. |
|
KER-3348 Decrease, AR activation leads to increase, nipple retention |
High |
High |
It is well established that activation of AR drives regression of nipple anlagen in males. The empirical evidence includes numerous in vivo toxicity studies showing that decreased AR activation leads to increased NR in male offspring, with few inconsistencies. The empirical evidence combined with theoretical considerations provide some support for dose, temporal, and incidence concordance for the KER, although this evidence is weak and indirect. |
Quantitative Consideration
The quantitative understanding of the AOP is limited. A key difficulty lies in the challenge of extrapolating from in vitro to in vivo events since these cannot be captured within the same experimental framework. Specifically, MIE-1617 is evaluated in vitro, while both KE-1613 (decrease, DHT levels’), KE-1614 (decrease, AR activation’) and the AO (Increase, NR) are in vivo endpoints. It should be noted that KE-1614 pertains to AR activation in vivo - currently lacking viable methods for direct measurement.
For in vivo to in vivo KERs like KER-1935 (‘Decrease, DHT level leads to Decrease, AR activation’) and KER-2124 (‘Decrease, AR activation leads to Altered, Transcription of genes by the AR’), there is not enough data to define a quantitative relationship, and such a relationship will differ between biological systems (species, tissue, cell type, life stage etc).
Considerations for Potential Applications of the AOP (optional)
The AOP supports the regulatory application of NR as a measure of endocrine disruption relevant for human health and the use of NR as an indicator of anti-androgenicity in mammals and other vertebrates in the environment.
NR is a mandatory endpoint in multiple OECD test guidelines, including TG 443 (extended one-generation reproductive toxicity study) and TGs 421/422 (reproductive toxicity screening studies) (OECD 2025a; OECD 2025b; OECD 2025c). NR can contribute to establishing a No Observed Adverse Effect Level (NOAEL), as outlined in OECD guidance documents No. 43 and 151 (OECD 2008; OECD 2013). The ability to derive a NOAEL for increased NR in male rodent offspring, which can serve as a point of departure for determining human safety thresholds, underscores the regulatory significance of this AOP.
The AOP also holds utility for informing on anti-androgenicity more generally, as this modality is highly relevant across mammalian species (Schwartz et al., 2021) and vertebrates more broadly due to the conserved nature of the AR and its implication in sexual differentiation across species (Ogino et al., 2023).
References
Aggarwal, S., Thareja, S., Verma, A., Bhardwaj, T. R., & Kumar, M. (2010). An overview on 5α-reductase inhibitors. Steroids, 75(2), 109–153. https://doi.org/10.1016/j.steroids.2009.10.005
Amato, C. M., Yao, H. H.-C., & Zhao, F. (2022). One Tool for Many Jobs: Divergent and Conserved Actions of Androgen Signaling in Male Internal Reproductive Tract and External Genitalia. Frontiers in Endocrinology, 13. https://doi.org/10.3389/fendo.2022.910964
Bologna, M., Muzi, P., Biordi, L., Festuccia, C., & Vicentini, C. (1995). Finasteride dose-dependently reduces the proliferation rate of the LnCap human prostatic cancer cell line in vitro. Urology, 45(2), 282–290. https://doi.org/10.1016/0090-4295(95)80019-0
Chamberlain, N. L., Driver, E. D., & Miesfeld, R. L. (1994). The length and location of CAG trinucleotide repeats in the androgen receptor N-terminal domain affect transactivation function. Nucleic Acids Research, 22(15), 3181–3186. https://doi.org/10.1093/nar/22.15.3181
Christiansen S, Scholze M, Dalgaard M, Vinggaard AM, Axelstad M, Kortenkamp A, & Hass U. (2009). Synergistic disruption of external male sex organ development by a mixture of four antiandrogens. Environmental Health Perspectives, 117(12), 1839–1846. https://doi.org/10.1289/ehp.0900689
Clark, R. V., Hermann, D. J., Cunningham, G. R., Wilson, T. H., Morrill, B. B., & Hobbs, S. (2004). Marked Suppression of Dihydrotestosterone in Men with Benign Prostatic Hyperplasia by Dutasteride, a Dual 5α-Reductase Inhibitor. The Journal of Clinical Endocrinology & Metabolism, 89(5), 2179–2184. https://doi.org/10.1210/jc.2003-030330
Davey, R. A., & Grossmann, M. (2016). Androgen Receptor Structure, Function and Biology: From Bench to Bedside. The Clinical Biochemist. Reviews, 36(4), 3–15.
Drake, L., Hordinsky, M., Fiedler, V., Swinehart, J., Unger, W. P., Cotterill, P. C., Thiboutot, D. M., Lowe, N., Jacobson, C., Whiting, D., Stieglitz, S., Kraus, S. J., Griffin, E. I., Weiss, D., Carrington, P., Gencheff, C., Cole, G. W., Pariser, D. M., Epstein, E. S., … Waldstreicher, J. (1999). The effects of finasteride on scalp skin and serum androgen levels in men with androgenetic alopecia. Journal of the American Academy of Dermatology, 41(4), 550–554.
Draskau, M. K., Rosenmai, A. K., Bouftas, N., Johansson, H. K. L., Panagiotou, E. M., Holmer, M. L., Elmelund, E., Zilliacus, J., Beronius, A., Damdimopoulou, P., van Duursen, M., & Svingen, T. (2024). AOP Report: An Upstream Network for Reduced Androgen Signaling Leading to Altered Gene Expression of Androgen Receptor–Responsive Genes in Target Tissues. Environmental Toxicology and Chemistry, 43(11), 2329–2337. https://doi.org/10.1002/etc.5972
Goldman AS, Shapiro B, & Neumann F. (1976). Role of testosterone and its metabolites in the differentiation of the mammary gland in rats. Endocrinology, 99(6), 1490–1495. https://doi.org/10.1210/endo-99-6-1490
Holmer ML, Zilliacus J, Draskau MK, Hlisníková H, Beronius A, Svingen T. Methodology for developing data-rich Key Event Relationships for Adverse Outcome Pathways exemplified by linking decreased androgen receptor activity with decreased anogenital distance. Reprod Toxicol. 2024 Sep;128:108662. doi: 10.1016/j.reprotox.2024.108662 . Epub 2024 Jul 8. PMID: 38986849.
Imperato-McGinley J, Binienda Z, Gedney J, & Vaughan ED Jr. (1986). Nipple differentiation in fetal male rats treated with an inhibitor of the enzyme 5 alpha-reductase: definition of a selective role for dihydrotestosterone. Endocrinology, 118(1), 132–137. https://doi.org/10.1210/endo-118-1-132
Knapczyk-Stwora, K., Nynca, A., Ciereszko, R. E., Paukszto, L., Jastrzebski, J. P., Czaja, E., Witek, P., Koziorowski, M., & Slomczynska, M. (2019). Flutamide-induced alterations in transcriptional profiling of neonatal porcine ovaries. Journal of Animal Science and Biotechnology, 10(1), 35. https://doi.org/10.1186/s40104-019-0340-y
Kratochwil, K., & Schwartz, P. (1976). Tissue interaction in androgen response of embryonic mammary rudiment of mouse: identification of target tissue for testosterone. Proceedings of the National Academy of Sciences, 73(11), 4041–4044. https://doi.org/10.1073/pnas.73.11.4041
Leung, J. K., & Sadar, M. D. (2017). Non-Genomic Actions of the Androgen Receptor in Prostate Cancer. Frontiers in Endocrinology, 8. https://doi.org/10.3389/fendo.2017.00002
Mendonca, B. B., Batista, R. L., Domenice, S., Costa, E. M. F., Arnhold, I. J. P., Russell, D. W., & Wilson, J. D. (2016). Steroid 5α-reductase 2 deficiency. The Journal of Steroid Biochemistry and Molecular Biology, 163, 206–211. https://doi.org/10.1016/j.jsbmb.2016.05.020
OECD (2008), Guidance Document on Mammalian Reproductive Toxicity Testing and Assessment, OECD Series on Testing and Assessment, No. 43, OECD Publishing, Paris, https://doi.org/10.1787/d2631d22-en.
OECD (2013), Guidance Document Supporting OECD Test Guideline 443 on the Extended One-Generational Reproductive Toxicity Test, OECD Series on Testing and Assessment, No. 151, OECD Publishing, Paris, ENV/JM/MONO(2013)10
OECD. (2023). Test No. 458: Stably Transfected Human Androgen Receptor Transcriptional Activation Assay for Detection of Androgenic Agonist and Antagonist Activity of Chemicals. OECD Publishing. https://doi.org/10.1787/9789264264366-en
OECD. (2025a). Test No. 421: Reproduction/Developmental Toxicity Screening Test. https://doi.org/10.1787/9789264264380-en
OECD. (2025b). Test No. 422: Combined Repeated Dose Toxicity Study with the Reproduction/Developmental Toxicity Screening Test. https://doi.org/10.1787/9789264264403-en
OECD. (2025c). Test No. 443: Extended One-Generation Reproductive Toxicity Study. https://doi.org/10.1787/9789264185371-en
Ogino, Y., Ansai, S., Watanabe, E. et al. Evolutionary differentiation of androgen receptor is responsible for sexual characteristic development in a teleost fish. Nat Commun 14, 1428 (2023). https://doi.org/10.1038/s41467-023-37026-6
Pedersen, E. B., Christiansen, S., & Svingen, T. (2022). AOP key event relationship report: Linking androgen receptor antagonism with nipple retention. In Current Research in Toxicology (Vol. 3). Elsevier B.V. https://doi.org/10.1016/j.crtox.2022.100085
Robitaille, J., & Langlois, V. S. (2020). Consequences of steroid-5α-reductase deficiency and inhibition in vertebrates. General and Comparative Endocrinology, 290, 113400. https://doi.org/10.1016/j.ygcen.2020.113400
Schwartz, C. L., Christiansen, S., Hass, U., Ramhøj, L., Axelstad, M., Löbl, N. M., & Svingen, T. (2021). On the Use and Interpretation of Areola/Nipple Retention as a Biomarker for Anti-androgenic Effects in Rat Toxicity Studies. In Frontiers in Toxicology (Vol. 3). Frontiers Media S.A. https://doi.org/10.3389/ftox.2021.730752
Svingen, T., Villeneuve, D. L., Knapen, D., Panagiotou, E. M., Draskau, M. K., Damdimopoulou, P., & O’Brien, J. M. (2021). A Pragmatic Approach to Adverse Outcome Pathway Development and Evaluation. Toxicological Sciences, 184(2), 183–190. https://doi.org/10.1093/toxsci/kfab113
Tut, T. G., Ghadessy, F. J., Trifiro, M. A., Pinsky, L., & Yong, E. L. (1997). Long Polyglutamine Tracts in the Androgen Receptor Are Associated with Reduced Trans -Activation, Impaired Sperm Production, and Male Infertility 1. The Journal of Clinical Endocrinology & Metabolism, 82(11), 3777–3782. https://doi.org/10.1210/jcem.82.11.4385
Williams, A. J., Grulke, C. M., Edwards, J., McEachran, A. D., Mansouri, K., Baker, N. C., Patlewicz, G., Shah, I., Wambaugh, J. F., Judson, R. S., & Richard, A. M. (2017). The CompTox Chemistry Dashboard: a community data resource for environmental chemistry. Journal of Cheminformatics, 9(1), 61. https://doi.org/10.1186/s13321-017-0247-6
Wolf, C., Lambright, C., Mann, P., Price, M., Cooper, R. L., Ostby, J., & Earl Gray, L. J. (1999). Administration of potentially antiandrogenic pesticides (procymidone, linuron, iprodione, chlozolinate, p,p-DDE, and ketoconazole) and toxic substances (dibutyl-and diethylhexyl phthalate, PCB 169, and ethane dimethane sulphonate) during sexual differentiation produces diverse profiles of reproductive malformations in the male rat. Toxicology and Industrial Health, 15, 94–118. www.stockton-press.co.uk
You, L., Casanova, M., Archibeque-Engle, S., Sar, M., Fan, L.-Q., & Heck, A. (1998). Impaired Male Sexual Development in Perinatal Sprague-Dawley and Long-Evans Hooded Rats Exposed in Utero and Lactationally to p,p’-DDE. In TOX1COLOGICAL SCIENCES (Vol. 45). https://academic.oup.com/toxsci/article/45/2/162/1653877
Appendix 1
List of MIEs in this AOP
Event: 1617: Inhibition, 5α-reductase
Short Name: Inhibition, 5α-reductase
AOPs Including This Key Event
| AOP ID and Name | Event Type |
|---|---|
| Aop:289 - Inhibition of 5α-reductase leading to impaired fecundity in female fish | MolecularInitiatingEvent |
| Aop:305 - 5α-reductase inhibition leading to short anogenital distance (AGD) in male (mammalian) offspring | MolecularInitiatingEvent |
| Aop:120 - Inhibition of 5α-reductase leading to Leydig cell tumors (in rat) | MolecularInitiatingEvent |
| Aop:571 - 5α-reductase inhibition leading to hypospadias in male (mammalian) offspring | MolecularInitiatingEvent |
| Aop:576 - 5α-reductase inhibition leading to increased nipple retention (NR) in male (rodent) offspring | MolecularInitiatingEvent |
Biological Context
| Level of Biological Organization |
|---|
| Molecular |
Cell term
| Cell term |
|---|
| eukaryotic cell |
Domain of Applicability
Taxonomic Applicability| Term | Scientific Term | Evidence | Links |
|---|---|---|---|
| mammals | mammals | High | NCBI |
| Life Stage | Evidence |
|---|---|
| During development and at adulthood | High |
| Sex | Evidence |
|---|---|
| Mixed | High |
This KE is applicable to both sexes, across developmental stages into adulthood, in many different tissues and across mammalian taxa. It is, however, acknowledged that this KE most likely has a much broader domain of applicability extending to non-mammalian vertebrates. AOP developers are encouraged to add additional relevant knowledge to expand on the applicability to also include other vertebrates.
Essentially the reaction performed by the isozymes is the same, but the enzyme is differentially expressed in the body. 5α-reductase type 1 is mainly linked to the production of neurosteroids, 5α-reductase type 2 is mainly involved in production of 5α-DHT, whereas 5α-reductase type 3 is involved in N-glycosylation (Robitaille & Langlois, 2020).
The expression profile of the three 5α-reductase isoforms depends on the developmental stage, the tissue of interest, and the disease state of the tissue. The enzymes have been identified in, for instance, non-genital and genital skin, scalp, prostate, liver, seminal vesicle, epididymis, testis, ovary, kidney, exocrine pancreas, and brain (Azzouni, 2012, Uhlen 2015).
5α-reductase is well-conserved, all primary species in Eukaryota contain all three isoforms (from plant, amoeba, yeast to vertebrates) (Azzouni, 2012) and the enzymes are expressed in both males and females (Langlois, 2010, Uhlen 2015).
Key Event Description
This KE describes the inhibition of 5α-reductases (3-oxo-5α-steroid 4-dehydrogenases). These enzymes are widely expressed in tissues of both sexes and responsible for conversion of steroid hormones.
There are three isozymes: 5α-reductase type 1, 2, and 3. The substrates for 5α-reductases are 3-oxo (3-keto), Δ4,5 C19/C21 steroids such as testosterone, progesterone, androstenedione, epi-testosterone, cortisol, aldosterone, and deoxycorticosterone. The enzymatic reaction leads to an irreversible breakage of the double bond between carbon 4 and 5 and subsequent insertion of a hydride anion at carbon 5 and insertion of a proton at carbon 4. The reaction is aided by the cofactor NADPH. The substrate affinity and reaction velocity differ depending on the combination of substrate and enzyme isoform, for instance 5α-reductase type 2 has a higher substrate affinity for testosterone than the type 1 isoform of the enzyme, and the enzymatic reaction occurs at a higher velocity under optimal conditions. Likewise, inhibitors of 5α-reductase may exhibit differential effects depending on isoforms (Azzouni et al., 2012).
How it is Measured or Detected
There is currently (as of 2023) no OECD test guideline for the measurement of 5α-reductase inhibition.
Assessing the ability of chemicals to inhibit the activity of 5α-reductase is challenging, but has been assessed using transfected cell lines. This has been demonstrated in HEK-293 cells stably transfected with human 5α-reductase type 1, 2, and 3 (Yamana et al., 2010), in CHO cells stably transfected with human 5α-reductase type 1 and 2 (Thigpens et al., 1993), and COS cells transfected with human and rat 5α-reductase with unspecified isoforms (Andersson & Russell, 1990). The transfected cells are typically used as intact cells or cell homogenates. Further, 5α-reductase 1 and 2 has been successfully expressed and isolated from Escherichia coli with subsequent functionality allowing for examination of enzyme inhibition (Peng et al., 2020). The availability of the stably transfected cell lines and the isolated enzymes to the scientific community is unknown.
The output of the above methods could be decreased dihydrotestosterone (DHT) with increasing test chemical concentrations. Other substrates exist for the different isoforms that could be used to assess the enzymatic inhibition (Peng et al., 2020). The use of radiolabeled steroids has historic and continued use for 5α-reductase inhibition examination (Andersson & Russell, 1990; Peng et al., 2020; Thigpens et al., 1993; Yamana et al., 2010); however, alternative methods are available, such as conventional ELISA kits or advanced analytical methods such as liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS).
References
Andersson, S., & Russell, D. W. (1990). Structural and biochemical properties of cloned and expressed human and rat steroid 5a-reductases. Proc. Natl. Acad. Sci. USA, 87, 3640–3644. https://www.pnas.org
Azzouni, F., Godoy, A., Li, Y., & Mohler, J. (2012). The 5 alpha-reductase isozyme family: A review of basic biology and their role in human diseases. In Advances in Urology. https://doi.org/10.1155/2012/530121
Peng, H. M., Valentin-Goyco, J., Im, S. C., Han, B., Liu, J., Qiao, J., & Auchus, R. J. (2020). Expression in escherichia coli, purification, and functional reconstitution of human steroid 5α-reductases. Endocrinology (United States), 161(8), 1–11. https://doi.org/10.1210/ENDOCR/BQAA117
Robitaille, J., & Langlois, V. S. (2020). Consequences of steroid-5α-reductase deficiency and inhibition in vertebrates. In General and Comparative Endocrinology (Vol. 290). Academic Press Inc. https://doi.org/10.1016/j.ygcen.2020.113400
Thigpens, A. E., Cala, K. M., & Russell, D. W. (1993). Characterization of Chinese Hamster Ovary Cell Lines Expressing Human Steroid 5a-Reductase Isozymes. The Journal of Biological Chemistry, 268(23), 17404–17412.
Yamana, K., Fernand, L., Luu-The, V., & Luu-The, V. (2010). Human type 3 5α-reductase is expressed in peripheral tissues at higher levels than types 1 and 2 and its activity is potently inhibited by finasteride and dutasteride. Hormone Molecular Biology and Clinical Investigation, 2(3), 293–299. https://doi.org/10.1515/HMBCI.2010.035
List of Key Events in the AOP
Event: 1613: Decrease, dihydrotestosterone (DHT) levels
Short Name: Decrease, DHT level
Key Event Component
| Process | Object | Action |
|---|---|---|
| hormone biosynthetic process | 17beta-Hydroxy-2-oxa-5alpha-androstan-3-one | decreased |
AOPs Including This Key Event
Biological Context
| Level of Biological Organization |
|---|
| Tissue |
Domain of Applicability
Taxonomic Applicability| Term | Scientific Term | Evidence | Links |
|---|---|---|---|
| mammals | mammals | High | NCBI |
| Life Stage | Evidence |
|---|---|
| All life stages | Moderate |
| Sex | Evidence |
|---|---|
| Mixed | High |
This KE is applicable to both sexes, across developmental stages and adulthood, in many different tissues and across mammals.
In both humans and rodents, DHT is important for the in utero differentiation and growth of the prostate and male external genitalia (Azzouni et al., 2012; Gerald & Raj, 2022). Besides its critical role in development, DHT also induces growth of facial and body hair during puberty in humans (Azzouni et al., 2012).
In mammals, the role of DHT in females is less established (Swerdloff et al., 2017), however studies suggest that androgens are important in e.g. bone metabolism and growth, as well as female reproduction from follicle development to parturition (Hammes & Levin, 2019).
It is, however, acknowledged that this KE most likely has a much broader domain of applicability extending to non-mammalian vertebrates. AOP developers are encouraged to add additional relevant knowledge to expand on the applicability to also include other vertebrates.
Key Event Description
Dihydrotestosterone (DHT) is an endogenous steroid hormone and a potent androgen. The level of DHT in tissue or blood is dependent on several factors, such as the synthesis, uptake/release, metabolism, and elimination from the system, which again can be dependent on biological compartment and developmental stage.
DHT is primarily synthesized from testosterone (T) via the irreversible enzymatic reaction facilitated by 5α-Reductases (5α-REDs) (Swerdloff et al., 2017). Different isoforms of this enzyme are differentially expressed in specific tissues (e.g. prostate, skin, liver, and hair follicles) at different developmental stages, and depending on disease status (Azzouni et al., 2012; Uhlén et al., 2015), which ultimately affects the local production of DHT.
An alternative (“backdoor”) pathway , exists for DHT formation that is independent of T and androstenedione as precursors. While first discovered in marsupials, the physiological importance of this pathway has now also been established in other mammals including humans (Renfree and Shaw, 2023). This pathway relies on the conversion of progesterone (P) or 17-OH-P to androsterone and then androstanediol through several enzymatic reactions and finally, the conversion of androstanediol into DHT probably by HSD17B6 (Miller & Auchus, 2019; Naamneh Elzenaty et al., 2022). The “backdoor” synthesis pathway is a result of an interplay between placenta, adrenal gland, and liver during fetal life (Miller & Auchus, 2019).
The conversion of T to DHT by 5α-RED in peripheral tissue is mainly responsible for the circulating levels of DHT, though some tissues express enzymes needed for further metabolism of DHT consequently leading to little release and contribution to circulating levels (Swerdloff et al.).
The initial conversion of DHT into inactive steroids is primarily through 3α-hydroxysteroid dehydrogenase (3α-HSD) and 3β-HSD in liver, intestine, skin, and androgen-sensitive tissues. The subsequent conjugation is mainly mediated by uridine 5´-diphospho (UDP)-glucuronyltransferase 2 (UGT2) leading to biliary and urinary elimination from the system. Conjugation also occurs locally to control levels of highly potent androgens (Swerdloff et al., 2017).
Disruption of any of the aforementioned processes may lead to decreased DHT levels, either systemically or at tissue level.
How it is Measured or Detected
Several methods exist for DHT identification and quantification, such as conventional immunoassay methods (ELISA or RIA) and advanced analytical methods as liquid chromatography tandem mass spectrometry (LC-MS/MS). The methods can have differences in detection and quantification limits, which should be considered depending on the DHT levels in the sample of interest. Further, the origin of the sample (e.g. cell culture, tissue, or blood) will have implications for the sample preparation.
Conventional immunoassays have limitations in that they can overestimate the levels of DHT compared to levels determined by gas chromatography mass spectrometry and liquid chromatography tandem mass spectrometry (Hsing et al., 2007; Shiraishi et al., 2008). This overestimation may be explained by lack of specificity of the DHT antibody used in the RIA and cross-reactivity with T in samples (Swerdloff et al., 2017).
Test guideline no. 456 (OECD 2023) uses a cell line, NCI-H295, capable of producing DHT at low levels. The test guideline is not validated for this hormone. Measurement of DHT levels in these cells require low detection and quantification limits. Any effect on DHT can be a result of many upstream molecular events that are specific for the NCI-H295 cells, and which may differ in other models for steroidogenesis.
References
Azzouni, F., Godoy, A., Li, Y., & Mohler, J. (2012). The 5 alpha-reductase isozyme family: A review of basic biology and their role in human diseases. In Advances in Urology. https://doi.org/10.1155/2012/530121
Gerald, T., & Raj, G. (2022). Testosterone and the Androgen Receptor. In Urologic Clinics of North America (Vol. 49, Issue 4, pp. 603–614). W.B. Saunders. https://doi.org/10.1016/j.ucl.2022.07.004
Hammes, S. R., & Levin, E. R. (2019). Impact of estrogens in males and androgens in females. In Journal of Clinical Investigation (Vol. 129, Issue 5, pp. 1818–1826). American Society for Clinical Investigation. https://doi.org/10.1172/JCI125755
Hsing, A. W., Stanczyk, F. Z., Bélanger, A., Schroeder, P., Chang, L., Falk, R. T., & Fears, T. R. (2007). Reproducibility of serum sex steroid assays in men by RIA and mass spectrometry. Cancer Epidemiology Biomarkers and Prevention, 16(5), 1004–1008. https://doi.org/10.1158/1055-9965.EPI-06-0792
Miller, W. L., & Auchus, R. J. (2019). The “backdoor pathway” of androgen synthesis in human male sexual development. PLoS Biology, 17(4). https://doi.org/10.1371/journal.pbio.3000198
Naamneh Elzenaty, R., du Toit, T., & Flück, C. E. (2022). Basics of androgen synthesis and action. In Best Practice and Research: Clinical Endocrinology and Metabolism (Vol. 36, Issue 4). Bailliere Tindall Ltd. https://doi.org/10.1016/j.beem.2022.101665
OECD (2023), Test No. 456: H295R Steroidogenesis Assay, OECD Guidelines for the Testing of Chemicals, Section 4, OECD Publishing, Paris, https://doi.org/10.1787/9789264122642-en.
Renfree, M. B., and Shaw, G. (2023). The alternate pathway of androgen metabolism and window of sensitivity. J. Endocrinol., JOE-22-0296. doi:10.1530/JOE-22-0296.
Shiraishi, S., Lee, P. W. N., Leung, A., Goh, V. H. H., Swerdloff, R. S., & Wang, C. (2008). Simultaneous measurement of serum testosterone and dihydrotestosterone by liquid chromatography-tandem mass spectrometry. Clinical Chemistry, 54(11), 1855–1863. https://doi.org/10.1373/clinchem.2008.103846
Swerdloff, R. S., Dudley, R. E., Page, S. T., Wang, C., & Salameh, W. A. (2017). Dihydrotestosterone: Biochemistry, physiology, and clinical implications of elevated blood levels. In Endocrine Reviews (Vol. 38, Issue 3, pp. 220–254). Endocrine Society. https://doi.org/10.1210/er.2016-1067
Uhlén, M., Fagerberg, L., Hallström, B. M., Lindskog, C., Oksvold, P., Mardinoglu, A., Sivertsson, Å., Kampf, C., Sjöstedt, E., Asplund, A., Olsson, I. M., Edlund, K., Lundberg, E., Navani, S., Szigyarto, C. A. K., Odeberg, J., Djureinovic, D., Takanen, J. O., Hober, S., … Pontén, F. (2015). Tissue-based map of the human proteome. Science, 347(6220). https://doi.org/10.1126/science.1260419
Event: 1614: Decrease, androgen receptor activation
Short Name: Decrease, AR activation
Key Event Component
| Process | Object | Action |
|---|---|---|
| androgen receptor activity | androgen receptor | decreased |
AOPs Including This Key Event
Biological Context
| Level of Biological Organization |
|---|
| Tissue |
Domain of Applicability
Taxonomic Applicability| Term | Scientific Term | Evidence | Links |
|---|---|---|---|
| mammals | mammals | High | NCBI |
| Life Stage | Evidence |
|---|---|
| During development and at adulthood | High |
| Sex | Evidence |
|---|---|
| Mixed | High |
This KE is considered broadly applicable across mammalian taxa as all mammals express the AR in numerous cells and tissues where it regulates gene transcription required for developmental processes and functions. It is, however, acknowledged that this KE most likely has a much broader domain of applicability extending to non-mammalian vertebrates. AOP developers are encouraged to add additional relevant knowledge to expand on the applicability to also include other vertebrates.
Key Event Description
This KE refers to decreased activation of the androgen receptor (AR) as occurring in complex biological systems such as tissues and organs in vivo. It is thus considered distinct from KEs describing either blocking of AR or decreased androgen synthesis.
The AR is a nuclear transcription factor with canonical AR activation regulated by the binding of the androgens such as testosterone or dihydrotestosterone (DHT). Thus, AR activity can be decreased by reduced levels of steroidal ligands (testosterone, DHT) or the presence of compounds interfering with ligand binding to the receptor (Davey & Grossmann, 2016; Gao et al., 2005).
In the inactive state, AR is sequestered in the cytoplasm of cells by molecular chaperones. In the classical (genomic) AR signaling pathway, AR activation causes dissociation of the chaperones, AR dimerization and translocation to the nucleus to modulate gene expression. AR binds to the androgen response element (ARE) (Davey & Grossmann, 2016; Gao et al., 2005). Notably, for transcriptional regulation the AR is closely associated with other co-factors that may differ between cells, tissues and life stages. In this way, the functional consequence of AR activation is cell- and tissue-specific. This dependency on co-factors such as the SRC proteins also means that stressors affecting recruitment of co-activators to AR can result in decreased AR activity (Heinlein & Chang, 2002).
Ligand-bound AR may also associate with cytoplasmic and membrane-bound proteins to initiate cytoplasmic signaling pathways with other functions than the nuclear pathway. Non-genomic AR signaling includes association with Src kinase to activate MAPK/ERK signaling and activation of the PI3K/Akt pathway. Decreased AR activity may therefore be a decrease in the genomic and/or non-genomic AR signaling pathways (Leung & Sadar, 2017).
How it is Measured or Detected
This KE specifically focuses on decreased in vivo activation, with most methods that can be used to measure AR activity carried out in vitro. They provide indirect information about the KE and are described in lower tier MIE/KEs (see for example MIE/KE-26 for AR antagonism, KE-1690 for decreased T levels and KE-1613 for decreased dihydrotestosterone levels). Assays may in the future be developed to measure AR activation in mammalian organisms.
References
Davey, R. A., & Grossmann, M. (2016). Androgen Receptor Structure, Function and Biology: From Bench to Bedside. The Clinical Biochemist. Reviews, 37(1), 3–15.
Gao, W., Bohl, C. E., & Dalton, J. T. (2005). Chemistry and structural biology of androgen receptor. Chemical Reviews, 105(9), 3352–3370. https://doi.org/10.1021/cr020456u
Heinlein, C. A., & Chang, C. (2002). Androgen Receptor (AR) Coregulators: An Overview. https://academic.oup.com/edrv/article/23/2/175/2424160
Leung, J. K., & Sadar, M. D. (2017). Non-Genomic Actions of the Androgen Receptor in Prostate Cancer. Frontiers in Endocrinology, 8. https://doi.org/10.3389/fendo.2017.00002
OECD (2022). Test No. 251: Rapid Androgen Disruption Activity Reporter (RADAR) assay. Paris: OECD Publishing doi:10.1787/da264d82-en.
|
|
|
Event: 286: Altered, Transcription of genes by the androgen receptor
Short Name: Altered, Transcription of genes by the AR
Key Event Component
| Process | Object | Action |
|---|---|---|
| regulation of gene expression | androgen receptor | decreased |
AOPs Including This Key Event
Stressors
| Name |
|---|
| Bicalutamide |
| Cyproterone acetate |
| Epoxiconazole |
| Flutamide |
| Flusilazole |
| Prochloraz |
| Propiconazole |
| Stressor:286 Tebuconazole |
| Triticonazole |
| Vinclozalin |
Biological Context
| Level of Biological Organization |
|---|
| Tissue |
Domain of Applicability
Taxonomic Applicability| Term | Scientific Term | Evidence | Links |
|---|---|---|---|
| mammals | mammals | High | NCBI |
| Life Stage | Evidence |
|---|---|
| During development and at adulthood | High |
| Sex | Evidence |
|---|---|
| Mixed | High |
Both the DNA-binding and ligand-binding domains of the AR are highly evolutionary conserved, whereas the transactivation domain show more divergence, which may affect AR-mediated gene regulation across species (Davey and Grossmann 2016). Despite certain inter-species differences, AR function mediated through gene expression is highly conserved, with mutation studies from both humans and rodents showing strong correlation for AR-dependent development and function (Walters et al. 2010).
This KE is considered broadly applicable across mammalian taxa, sex and developmental stages, as all mammals express the AR in numerous cells and tissues where it regulates gene transcription required for developmental processes and function. It is, however, acknowledged that this KE most likely has a much broader domain of applicability extending to non-mammalian vertebrates. AOP developers are encouraged to add additional relevant knowledge to expand on the applicability to also include other vertebrates.
Key Event Description
This KE refers to transcription of genes by the androgen receptor (AR) as occurring in complex biological systems such as tissues and organs in vivo. Rather than measuring individual genes, this KE aims to capture patterns of effects at transcriptome level in specific target cells/tissues. In other words, it can be replaced by specific KEs for individual adverse outcomes as information becomes available, for example the transcriptional toxicity response in prostate tissue for AO: prostate cancer, perineum tissue for AO: reduced AGD, etc. AR regulates many genes that differ between tissues and life stages and, importantly, different gene transcripts within individual cells can go in either direction since AR can act as both transcriptional activator and suppressor. Thus, the ‘directionality’ of the KE cannot be either reduced or increased, but instead describe an altered transcriptome.
The Androgen Receptor and its function
The AR belongs to the steroid hormone nuclear receptor family. It is a ligand-activated transcription factor with three domains: the N-terminal domain, the DNA-binding domain, and the ligand-binding domain with the latter being the most evolutionary conserved (Davey and Grossmann 2016). Androgens (such as dihydrotestosterone and testosterone) are AR ligands and act by binding to the AR in androgen-responsive tissues (Davey and Grossmann 2016). Human AR mutations and mouse knockout models have established a fundamental role for AR in masculinization and spermatogenesis (Maclean et al.; Walters et al. 2010; Rana et al. 2014). The AR is also expressed in many other tissues such as bone, muscles, ovaries and within the immune system (Rana et al. 2014).
Altered transcription of genes by the AR as a Key Event
Upon activation by ligand-binding, the AR translocates from the cytoplasm to the cell nucleus, dimerizes, binds to androgen response elements in the DNA to modulate gene transcription (Davey and Grossmann 2016). The transcriptional targets vary between cells and tissues, as well as with developmental stages and is also dependent on available co-regulators (Bevan and Parker 1999; Heemers and Tindall 2007). It should also be mentioned that the AR can work in other ‘non-canonial’ ways such as non-genomic signaling, and ligand-independent activation (Davey & Grossmann, 2016; Estrada et al, 2003; Jin et al, 2013).
A large number of known, and proposed, target genes of AR canonical signaling have been identified by analysis of gene expression following treatments with AR agonists (Bolton et al. 2007; Ngan et al. 2009, Jin et al. 2013).
How it is Measured or Detected
Altered transcription of genes by the AR can be measured by measuring the transcription level of known downstream target genes by RT-qPCR or other transcription analyses approaches, e.g. transcriptomics.
Since this KE aims to capture AR-mediated transcriptional patterns of effect, downstream bioinformatics analyses will typically be required to identify and compare effect footprints. Clusters of genes can be statistically associated with, for example, biological process terms or gene ontology terms relevant for AR-mediated signaling. Large transcriptomics data repositories can be used to compare transcriptional patterns between chemicals, tissues, and species (e.g. TOXsIgN (Darde et al, 2018a; Darde et al, 2018b), comparisons can be made to identified sets of AR ‘biomarker’ genes (e.g. as done in (Rooney et al, 2018)), and various methods can be used e.g. connectivity mapping (Keenan et al, 2019).
References
Bevan C, Parker M (1999) The role of coactivators in steroid hormone action. Exp. Cell Res. 253:349–356
Bolton EC, So AY, Chaivorapol C, et al (2007) Cell- and gene-specific regulation of primary target genes by the androgen receptor. Genes Dev 21:2005–2017. doi: 10.1101/gad.1564207
Darde, T. A., Gaudriault, P., Beranger, R., Lancien, C., Caillarec-Joly, A., Sallou, O., et al. (2018a). TOXsIgN: a cross-species repository for toxicogenomic signatures. Bioinformatics 34, 2116–2122. doi:10.1093/bioinformatics/bty040.
Darde, T. A., Chalmel, F., and Svingen, T. (2018b). Exploiting advances in transcriptomics to improve on human-relevant toxicology. Curr. Opin. Toxicol. 11–12, 43–50. doi:10.1016/j.cotox.2019.02.001.
Davey RA, Grossmann M (2016) Androgen Receptor Structure, Function and Biology: From Bench to Bedside. Clin Biochem Rev 37:3–15
Estrada M, Espinosa A, Müller M, Jaimovich E (2003) Testosterone Stimulates Intracellular Calcium Release and Mitogen-Activated Protein Kinases Via a G Protein-Coupled Receptor in Skeletal Muscle Cells. Endocrinology 144:3586–3597. doi: 10.1210/en.2002-0164
Heemers H V., Tindall DJ (2007) Androgen receptor (AR) coregulators: A diversity of functions converging on and regulating the AR transcriptional complex. Endocr. Rev. 28:778–808
Jin, Hong Jian, Jung Kim, and Jindan Yu. 2013. “Androgen Receptor Genomic Regulation.” Translational Andrology and Urology 2(3):158–77. doi: 10.3978/j.issn.2223-4683.2013.09.01
Keenan, A. B., Wojciechowicz, M. L., Wang, Z., Jagodnik, K. M., Jenkins, S. L., Lachmann, A., et al. (2019). Connectivity Mapping: Methods and Applications. Annu. Rev. Biomed. Data Sci. 2, 69–92. doi:10.1146/ANNUREV-BIODATASCI-072018-021211.
Maclean HE, Chu S, Warne GL, Zajact JD Related Individuals with Different Androgen Receptor Gene Deletions
MacLeod DJ, Sharpe RM, Welsh M, et al (2010) Androgen action in the masculinization programming window and development of male reproductive organs. In: International Journal of Andrology. Blackwell Publishing Ltd, pp 279–287
Ngan S, Stronach EA, Photiou A, et al (2009) Microarray coupled to quantitative RT–PCR analysis of androgen-regulated genes in human LNCaP prostate cancer cells. Oncogene 28:2051–2063. doi: 10.1038/onc.2009.68
Rana K, Davey RA, Zajac JD (2014) Human androgen deficiency: Insights gained from androgen receptor knockout mouse models. Asian J. Androl. 16:169–177
Rooney, J. P., Chorley, B., Kleinstreuer, N., and Corton, J. C. (2018). Identification of Androgen Receptor Modulators in a Prostate Cancer Cell Line Microarray Compendium. Toxicol. Sci. 166, 146–162. doi:10.1093/TOXSCI/KFY187.
Walters KA, Simanainen U, Handelsman DJ (2010) Molecular insights into androgen actions in male and female reproductive function from androgen receptor knockout models. Hum Reprod Update 16:543–558. doi: 10.1093/humupd/dmq003
List of Adverse Outcomes in this AOP
Event: 1786: Nipple retention (NR), increased
Short Name: nipple retention, increased
AOPs Including This Key Event
Biological Context
| Level of Biological Organization |
|---|
| Individual |
Domain of Applicability
Taxonomic Applicability Life Stage Applicability| Life Stage | Evidence |
|---|---|
| Birth to < 1 month | High |
| Sex | Evidence |
|---|---|
| Male | High |
The applicability domain of NR is limited to male laboratory strains of rats and mice from birth to juvenile age.
Key Event Description
In common laboratory strains of rats and mice, females typically have 6 (rats) or 5 (mice) pairs of nipples along the bilateral milk lines. In contrast, male rats and mice do not have nipples. This is unlike e.g., humans where both sexes have 2 nipples (Schwartz et al., 2021).
In laboratory rats, high levels of dihydrotestosterone (DHT) induce regression of the nipples in males (Imperato-McGinley & Gautier, 1986; Kratochwil, 1977; Kratochwil & Schwartz, 1976). Females, in the absence of this DHT surge, retain their nipples. This relationship has also been shown in numerous rat studies with perinatal exposure to anti-androgenic chemicals (Schwartz et al., 2021). Hence, if juvenile male rats and mice possess nipples, it is considered a sign of perturbed androgen action early in life.
This KE was first published by Pedersen et al (2022).
How it is Measured or Detected
Nipple retention (NR) is visually assessed, ideally on postnatal day (PND) 12/13 (OECD, 2018; Schwartz et al., 2021). However, PND 14 is also an accepted stage of examination (OECD, 2013). Depending on animal strain, the time when nipples become visible can vary, but the assessment of NR in males should be conducted when nipples are visible in their female littermates (OECD, 2013).
Nipples are detected as dark spots (or shadows) called areolae, which resemble precursors to a nipple rather than a fully developed nipple. The dark area may or may not display a nipple bud (Hass et al., 2007). Areolae typically emerge along the milk lines of the male pups corresponding to where female pups display nipples. Fur growth may challenge detection of areolae after PND 14/15. Therefore, the NR assessment should be conducted prior to excessive fur growth. Ideally, all pups in a study are assessed on the same postnatal day to minimize variation due to maturation level (OECD, 2013).
NR is occasionally observed in controls. Hence, accurate assessment of NR in controls is needed to detect substance-induced effects on masculine development (Schwartz et al., 2021). It is recommended by the OECD guidance documents 43 and 151 to record NR as a quantitative number rather than a qualitative measure (present/absent or yes/no response). This allows for more nuanced analysis of results, e.g., high control values may be recognized (OECD, 2013, 2018). Studies reporting quantitative measures of NR are therefore considered stronger in terms of weight of evidence.
Reproducibility of NR results is challenged by the measure being a visual assessment prone to a degree of subjectivity. Thus, NR should be assessed and scored blinded to exposure groups and ideally be performed by the same person(s) within the same study.
Regulatory Significance of the AO
NR is recognized by the OECD as a relevant measure for anti-androgenic effects and is mandatory in the test guidelines Extended One Generation Reproductive Toxicity Study, TG 443 (OECD, 2018) and the two screening studies for reproductive toxicity, TGs 421/422 (OECD, 2016a, 2016b). The endpoint is also described in the guidance documents 43 (OECD, 2008) and 151 (OECD, 2013). Furthermore, NR data can be used in chemical risk assessment for setting the No Observed Adverse Effect Level (NOAEL) as stated in the OECD guidance document 151 (OECD, 2013): “A statistically significant change in nipple retention should be evaluated similarly to an effect on AGD as both endpoints indicate an adverse effect of exposure and should be considered in setting a NOAEL”.
References
Hass, U., Scholze, M., Christiansen, S., Dalgaard, M., Vinggaard, A. M., Axelstad, M., Metzdorff, S. B., & Kortenkamp, A. (2007). Combined exposure to anti-androgens exacerbates disruption of sexual differentiation in the rat. Environmental Health Perspectives, 115(suppl 1), 122–128.
Imperato-McGinley, J., Binienda, Z., Gedney, J., & Vaughan, E. D. (1986). Nipple Differentiation in Fetal Male Rats Treated with an Inhibitor of the Enzyme 5α-Reductase: Definition of a Selective Role for Dihydrotestosterone. Endocrinology, 118(1), 132–137.
Kratochwil, K. (1977). Development and Loss of Androgen Responsiveness in the Embryonic Rudiment of the Mouse Mammary Gland. DEVELOPMENTAL BIOLOGY, 61, 358–365.
OECD. (2008). Guidance document 43 on mammalian reproductive toxicity testing and assessment. Environment, Health and Safety Publications, 16(43).
OECD. (2013). Guidance document supporting OECD test guideline 443 on the extended one-generation reproductive toxicity test. Environment, Health and Safety Publications, 10(151).
OECD. (2016a). Test Guideline 421: Reproduction/Developmental Toxicity Screening Test. OECD Guidelines for the Testing of Chemicals, 421. http://www.oecd.org/termsandconditions/
OECD. (2016b). Test Guideline 422: Combined Repeated Dose Toxicity Study with the Reproduction/Developmental Toxicity Screening Test. OECD Guidelines for the Testing of Chemicals, 422. http://www.oecd.org/termsandconditions/
OECD. (2018). Test Guideline 443: Extended one-generation reproductive toxicity study. OECD Guidelines for the Testing of Chemicals, 443. http://www.oecd.org/termsandconditions/
Pedersen, E. B., Christiansen, S., & Svingen, T. (2022). AOP key event relationship report: Linking androgen receptor antagonism with nipple retention. Current Research in Toxicology, 3, 100085.
Schwartz, C. L., Christiansen, S., Hass, U., Ramhøj, L., Axelstad, M., Löbl, N. M., & Svingen, T. (2021). On the Use and Interpretation of Areola/Nipple Retention as a Biomarker for Anti-androgenic Effects in Rat Toxicity Studies. Frontiers in Toxicology, 3, 730752.
Appendix 2
List of Key Event Relationships in the AOP
List of Adjacent Key Event Relationships
Relationship: 1880: Inhibition, 5α-reductase leads to Decrease, DHT level
AOPs Referencing Relationship
| AOP Name | Adjacency | Weight of Evidence | Quantitative Understanding |
|---|---|---|---|
| Inhibition of 5α-reductase leading to impaired fecundity in female fish | adjacent | High | High |
| 5α-reductase inhibition leading to short anogenital distance (AGD) in male (mammalian) offspring | adjacent | High | High |
| 5α-reductase inhibition leading to hypospadias in male (mammalian) offspring | adjacent | ||
| 5α-reductase inhibition leading to increased nipple retention (NR) in male (rodent) offspring | adjacent | High |
Evidence Supporting Applicability of this Relationship
| Term | Scientific Term | Evidence | Links |
|---|---|---|---|
| mammals | mammals | High | NCBI |
| Life Stage | Evidence |
|---|---|
| During development and at adulthood | High |
| Sex | Evidence |
|---|---|
| Mixed | High |
This KE is applicable for both sexes, across developmental stages into adulthood, in numerous cells and tissues and across mammalian taxa. It is, however, acknowledged that this KER most likely has a much broader domain of applicability extending to non-mammalian vertebrates. AOP developers are encouraged to add additional relevant knowledge to expand on the applicability to also include other vertebrates.
Key Event Relationship Description
This key event relationship (KER) links inhibition of 5α-reductase activity to decreased dihydrotestosterone (DHT) levels.
There are three isozymes of 5α-reductase: type 1, 2, and 3. 5α-reductase type 2 is mainly involved in the synthesis of 5α-DHT from testosterone (T) (Robitaille & Langlois, 2020), although 5α-reductase type 1 can also facilitate this reaction, but with lower affinity for T (Nikolaou et al., 2021). The type 1 isoform is also involved in the alternative (‘backdoor’) pathway for DHT formation, facilitating the conversion of progesterone or 17OH-progesterone to dihydroprogesterone or 5α-pregnan-17α-ol-3,20-dione, respectively, whereafter several subsequent reactions will ultimately lead to the formation of DHT (Miller & Auchus, 2019). The quantitative importance of the alternative pathway remains unclear (Alemany, 2022). The type 1 and type 2 isoforms of 5α-reductase are the primary focus of this KER.
The direct conversion of T to 5α-DHT mainly takes place in the target tissue (Robitaille & Langlois, 2020). In mammals, the type 1 isoform is found in the scalp and other peripheral tissues (Miller & Auchus, 2011), such as liver, skin, prostate (Azzouni et al., 2012), bone, ovaries, and adipose tissue (Nikolaou et al., 2021). The type 2 isoform is expressed mainly in male reproductive tissues (Miller & Auchus, 2011), but also in liver, scalp and skin (Nikolaou et al., 2021). The expression level of both isoforms depend on the developmental stage and the tissue.
Evidence Supporting this KER
Biological PlausibilityThe biological plausibility of this KER is considered high.
5α-reductase can catalyze the conversion of T to DHT. The substrates for 5α-reductases are 3-oxo (3-keto), Δ4,5 C19/C21 steroids such as testosterone and progesterone. The enzymatic reaction leads to an irreversible breakage of the double bond between carbon 4 and 5 and subsequent insertion of a hydride anion at carbon 5 and insertion of a proton at carbon 4. The reaction is aided by the cofactor NADPH (Azzouni et al., 2012). By inhibiting this enzyme, the described catalyzed reaction will be inhibited leading to a decrease in DHT levels.
In both humans and rodents, DHT is important for the in utero differentiation and growth of the prostate and male external genitalia. Besides its critical role during fetal development, DHT also induces growth of facial and body hair during puberty in humans (Azzouni et al., 2012).
Empirical EvidenceThe empirical evidence for this KER is considered high
Dose concordance
Several inhibitors of 5α-reductases have been developed for pharmacological uses. Inhibition of the enzymatic conversion of radiolabeled substrate has been illustrated (Table 1) and data display dose-concordance, with increasing concentrations of inhibitor leading to lower 5α-reductase product formation. These studies at large rely on conversion of radiolabeled substrate and hence serve as an indirect measurement.
Table 1: Dose concordance from selected in vitro test systems
|
Test system |
Model description |
Stressor |
Effect |
Reference |
|
HEK-293 cells |
Cells stably transfected human 5α-reductase type 1 and 2 used to measure conversion of [14C]labeled steroids |
Finasteride |
Type 1: IC50 = 106.9 µM Type 2: IC50 = 14.3 µM |
(Yamana et al., 2010) |
| Dutasteride |
Type 1: IC50 = 8.7 µM Type 2: IC50 = 57 µM |
|||
|
COS cells |
Cell homogenates from transfected cells with human and rat 5α-reductase (unknown isoform) used to measure conversion of radiolabeled testosterone |
Finasteride
|
Human: IC50 ≈ 1 µM Ki = 340-620 nM Rat: IC50 ≈ 0.1 µM Ki = 3-5 nM |
(Andersson & Russell, 1990) |
| 4-MA |
Human: IC50 ≈ 0.1 µM Ki = 7-8 nM Rat: IC50 ≈ 0.1 µM Ki = 5-7 nM |
|||
|
CHO cells |
Stably transfected with human 5α-reductase type 1 and 2 |
Finasteride
|
Type 1: Ki = 325 nM Type 2: Ki = 12 nM |
(Thigpens et al., 1993) |
| 4-MA |
Type 1: Ki = 8 nM Type 2: Ki = 4 nM |
|||
|
Isolated enzyme |
Human 5α-reductase type 1 and 2 used to measure conversion of radiolabeled substrate of both isoforms |
Finasteride |
Type 1: Ki = > 200 nM Type 2: Ki = 0.45 nM |
(Peng et al., 2020)
|
| Dutasteride |
Type 1: Ki = 39 nM Type 2: Ki = 1.1 nM |
These in vitro studies clearly show effects on the enzymatic reaction induced by 5α-reductases in a concentration dependent manner (Andersson & Russell, 1990; Thigpens et al., 1993; Yamana et al., 2010).
In the intact organism, when 5α-reductase type 2 activity is lacking through e.g. inhibitor treatment or knockout, this will results in decreased 5α-DHT locally in the tissues, but also in blood (Robitaille & Langlois, 2020). This has been demonstrated in humans, rats, monkeys, and mice (Robitaille et al. 2020).
Finasteride is a specific inhibitor of 5α-reductase type 2 (Russell & Wilson, 1994). Men with androgenic alopecia were treated with increasing concentrations of finasteride and presented with decreased DHT levels in biopsies from scalp, as well as a decrease in serum DHT levels with dose dependency being most apparent in serum, up to about 70% decrease (Drake et al., 1999). Likewise, men treated with dutasteride exhibited a clear dose dependent decrease in serum DHT after 24 weeks treatment with a maximum efficacy of about 98% (Clark et al., 2004).
Other evidence
The phenotype of males with deficiency in 5α-reductases are typically born with ambiguous external genitalia. They also present with small prostate, minimal facial hair and acne, or temporal hair loss. Comparison of affected individuals to non-affected individuals in regard to T/DHT ratio, conversion of infused radioactive T, and ratios of urinary metabolites of 5α-reductase and 5β-reductase concluded that these phenotypic characteristics were due to 5α-reductase defects that resulted in less conversion of T to DHT (Okeigwe et al. 2014). Mutations in the 5α-reductase gene can result in boys being born with moderate to severe undervirilization phenotypes (Elzenaty 2022).
Quantitative Understanding of the Linkage
Inhibitors of 5α-reductase are important for the prevention and treatment of many diseases. There are several compounds that have been developed for pharmaceutical purposes and they can target the different isoforms with different affinity. Examples of inhibitors are finasteride and dutasteride. Finasteride mainly has specificity for the type 2 isoform, whereas dutasteride inhibits both type 1 and 2 isoforms (Miller & Auchus, 2011).
These differences in isoform specificity reflects in the effects on DHT serum levels, hence the broader specificity of dutasteride leads to > 90% decrease in patients with benign prostatic hyperplasia, in comparison to 70% with finasteride administration (Nikolaou et al., 2021).
Response-response relationshipEnzyme inhibition can occur in different ways e.g. both competitive and noncompetitive. The inhibition model depends on the specific inhibitor and hence a generic quantitative response-response relationship is difficult to derive.
Time-scaleAn inhibition of 5α-reductases would lead to an immediate change in DHT levels at the molecular level. However, the time-scale for systemic effects on hormone levels are challenging to estimate.
Known Feedforward/Feedback loops influencing this KERAndrogens can regulate gene expression of 5α-reductases (Andersson et al., 1989; Berman & Russell, 1993).
References
Alemany, M. (2022). The Roles of Androgens in Humans: Biology, Metabolic Regulation and Health. In International Journal of Molecular Sciences (Vol. 23, Issue 19). MDPI. https://doi.org/10.3390/ijms231911952
Andersson, S., Bishop, R. W., & Russell$, D. W. (1989). THE JOURNAL OF BIOLOGICAL CHEMISTRY Expression Cloning and Regulation of Steroid 5cw-Reductase, an Enzyme Essential for Male Sexual Differentiation* (Vol. 264, Issue 27).
Andersson, S., & Russell, D. W. (1990). Structural and biochemical properties of cloned and expressed human and rat steroid 5a-reductases. Proc. Natl. Acad. Sci. USA, 87, 3640–3644. https://www.pnas.org
Azzouni, F., Godoy, A., Li, Y., & Mohler, J. (2012). The 5 alpha-reductase isozyme family: A review of basic biology and their role in human diseases. In Advances in Urology. https://doi.org/10.1155/2012/530121
Berman, D. M., & Russell, D. W. (1993). Cell-type-specific expression of rat steroid 5a-reductase isozymes (sexual development/androgens/prostate/stroma/epithelium). In Proc. Natl. Acad. Sci. USA (Vol. 90). https://www.pnas.org
Clark, R. V., Hermann, D. J., Cunningham, G. R., Wilson, T. H., Morrill, B. B., & Hobbs, S. (2004). Marked Suppression of Dihydrotestosterone in Men with Benign Prostatic Hyperplasia by Dutasteride, a Dual 5α-Reductase Inhibitor. Journal of Clinical Endocrinology and Metabolism, 89(5), 2179–2184. https://doi.org/10.1210/jc.2003-030330
Drake, L., Hordinsky, M., Fiedler, V., Swinehart, J., Unger, W. P., Cotterill, P. C., Thiboutot, D. M., Lowe, N., Jacobson, C., Whiting, D., Stieglitz, S., Kraus, S. J., Griffin, E. I., Weiss, D., Carrington, P., Gencheff, C., Cole, G. W., Pariser, D. M., Epstein, E. S., … City, O. (1999). The effects of finasteride on scalp skin and serum androgen levels in men with androgenetic alopecia.
Miller, W. L., & Auchus, R. J. (2011). The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocrine Reviews, 32(1), 81–151. https://doi.org/10.1210/er.2010-0013
Miller, W. L., & Auchus, R. J. (2019). The “backdoor pathway” of androgen synthesis in human male sexual development. PLoS Biology, 17(4). https://doi.org/10.1371/journal.pbio.3000198
Nikolaou, N., Hodson, L., & Tomlinson, J. W. (2021). The role of 5-reduction in physiology and metabolic disease: evidence from cellular, pre-clinical and human studies. In Journal of Steroid Biochemistry and Molecular Biology (Vol. 207). Elsevier Ltd. https://doi.org/10.1016/j.jsbmb.2021.105808
Peng, H. M., Valentin-Goyco, J., Im, S. C., Han, B., Liu, J., Qiao, J., & Auchus, R. J. (2020). Expression in escherichia coli, purification, and functional reconstitution of human steroid 5α-reductases. Endocrinology (United States), 161(8), 1–11. https://doi.org/10.1210/ENDOCR/BQAA117
Robitaille, J., & Langlois, V. S. (2020). Consequences of steroid-5α-reductase deficiency and inhibition in vertebrates. In General and Comparative Endocrinology (Vol. 290). Academic Press Inc. https://doi.org/10.1016/j.ygcen.2020.113400
Russell, D. W., & Wilson, J. D. (1994). STEROID Sa-REDUCTASE: TWO GENES/TWO ENZYMES. www.annualreviews.org
Thigpens, A. E., Cala, K. M., & Russell, D. W. (1993). Characterization of Chinese Hamster Ovary Cell Lines Expressing Human Steroid 5a-Reductase Isozymes. The Journal of Biological Chemistry, 268(23), 17404–17412.
Yamana, K., Fernand, L., Luu-The, V., & Luu-The, V. (2010). Human type 3 5α-reductase is expressed in peripheral tissues at higher levels than types 1 and 2 and its activity is potently inhibited by finasteride and dutasteride. Hormone Molecular Biology and Clinical Investigation, 2(3), 293–299. https://doi.org/10.1515/HMBCI.2010.035
Relationship: 1935: Decrease, DHT level leads to Decrease, AR activation
AOPs Referencing Relationship
| AOP Name | Adjacency | Weight of Evidence | Quantitative Understanding |
|---|---|---|---|
| Inhibition of 17α-hydrolase/C 10,20-lyase (Cyp17A1) activity leads to birth reproductive defects (cryptorchidism) in male (mammals) | adjacent | High | High |
| 5α-reductase inhibition leading to short anogenital distance (AGD) in male (mammalian) offspring | adjacent | High | |
| 5α-reductase inhibition leading to hypospadias in male (mammalian) offspring | adjacent | ||
| 5α-reductase inhibition leading to increased nipple retention (NR) in male (rodent) offspring | adjacent | High |
Evidence Supporting Applicability of this Relationship
| Term | Scientific Term | Evidence | Links |
|---|---|---|---|
| mammals | mammals | High | NCBI |
| Life Stage | Evidence |
|---|---|
| During development and at adulthood | High |
| Sex | Evidence |
|---|---|
| Mixed | High |
Taxonomic applicability
KER1935 is assessed applicable to mammals, as DHT and AR activation are known to be related in mammals. It is, however, acknowledged that this KER most likely has a much broader domain of applicability extending to non-mammalian vertebrates. AOP developers are encouraged to add additional relevant knowledge to expand on the applicability to also include other vertebrates.
Sex applicability
KER1935 is assessed applicable to both sexes, as DHT activates AR in both males and females.
Life-stage applicability
KER1935 is considered applicable to developmental and adult life stages, as DHT-mediated AR activation is relevant from the AR is expressed.
Key Event Relationship Description
Dihydrotestosterone (DHT) is a primary ligand for the Androgen receptor (AR), a nuclear receptor and transcription factor. DHT is an endogenous sex hormone that is synthesized from e.g. testosterone by the enzyme 5α-reductase in different tissues and organs (Davey & Grossmann, 2016; Marks, 2004). In the absence of ligand (e.g. DHT) the AR is localized in the cytoplasm in complex with molecular chaperones. Upon ligand binding, AR is activated, translocated into the nucleus, and dimerizes to carry out its ‘genomic function’ (Davey & Grossmann, 2016). Hence, AR transcriptional function is directly dependent on the presence of ligands, with DHT being a more potent AR activator than testosterone (Grino et al, 1990). Reduced levels of DHT may thus lead to reduced AR activation. Besides its genomic actions, the AR can also mediate rapid, non-genomic second messenger signaling (Davey and Grossmann, 2016). Decreased DHT levels that lead to reduced AR activation can thus entail downstream effects on both genomic and non-genomic signaling.
Evidence Supporting this KER
Biological PlausibilityThe biological plausibility of KER1935 is considered high.
The activation of AR is dependent on binding of ligands (though a few cases of ligand-independent AR activation has been shown, see uncertainties and inconsistencies), primarily testosterone and DHT in mammals (Davey and Grossmann, 2016; Schuppe et al., 2020). Without ligand activation, the AR will remain in the cytoplasm associated with heat-shock and other chaperones and not be able to carry out its canonical (‘genomic’) function. Upon androgen binding, the AR undergoes a conformational change, chaperones dissociate, and a nuclear localization signal is exposed. The androgen/AR complex can now translocate to the nucleus, dimerize and bind AR response elements to regulate target gene expression (Davey and Grossmann, 2016; Eder et al., 2001). AR transcriptional activity and specificity is regulated by co-activators and co-repressors in a cell-specific manner (Heinlein and Chang, 2002).
The requirement for androgens binding to the AR for transcriptional activity has been extensively studied and proven and is generally considered textbook knowledge. The OECD test guideline no. 458 uses DHT as the reference chemical for testing androgen receptor activation in vitro (OECD, 2020). In the absence of DHT during development caused by 5α-reductase deficiency (i.e. still in the presence of testosterone) male fetuses fail to masculinize properly. This is evidenced by, for instance, individuals with congenital 5α-reductase deficiency conditions (Costa et al., 2012); conditions not limited to humans (Robitaille and Langlois, 2020), testifying to the importance of specifically DHT for AR activation and subsequent masculinization of certain reproductive tissues.
Binding of testosterone or DHT has differential effects in different tissues. E.g. in the developing mammalian male; testosterone is required for development of the internal sex organs (epididymis, vas deferens and the seminal vesicles), whereas DHT is crucial for development of the external sex organs (Keller et al., 1996; Robitaille and Langlois, 2020).
Empirical EvidenceThe empirical support for KER1935 is considered high.
Dose concordance:
- Increasing concentrations of DHT lead to increasing AR activation in vitro in AR reporter gene assays (OECD, 2020; Williams et al., 2017).
Indirect (supporting) evidence:
- In cell lines where proliferation can be induced by androgens (such as prostate cancer cells) proliferation can be used as a readout for AR-activation. Finasteride, a 5α-reductase inhibitor, dose-dependently decreases AR-mediated prostate cancer cell line proliferation (Bologna et al., 1995). 0.001 µM finasteride decreased the growth rate with 44%, 0.1 µM decreased the growth rate with 80%.
- Specific events of masculinization during development are dependent on AR activation by DHT, including the development and length of the perineum which can be measured as the anogenital distance (AGD, (Schwartz et al., 2019)). E.g. a dose-dependent effect of rat in utero exposure to the 5α-reductase inhibitor finasteride was observed on the length of the AGD, where 0.01 mg/kg bw/day finasteride reduced the AGD measured at pup day 1 by 8%, whereas 1 mg/kg bw/day reduced the AGD by 23% (Bowman et al., 2003).
Other evidence:
- Male individuals with congenital 5α-reductase deficiency (absence of DHT) fail to masculinize properly (Costa et al., 2012).
- A major driver of prostate cancer growth is AR activation (Davey and Grossmann, 2016; Huggins and Hodges, 1941). Androgen deprivation is used as treatment including 5α-reductase inhibitors to reduce DHT levels (Aggarwal et al., 2010).
Ligand-independent actions of the AR have been identified. To what extent and of which biological consequences is not well defined (Bennesch and Picard, 2015).
It should be noted, that in tissues, that are not DHT-dependent but rather respond to T, a decrease in DHT level may not influence AR activation significantly in that specific tissue.
Quantitative Understanding of the Linkage
Response-response relationshipThere is a positive dose-response relationship between increasing concentrations of DHT and AR activation (Dalton et al., 1998; OECD, 2020). However, there is not enough data, or overview of the data, to define a quantitative linkage in vivo, and such a relationship will differ between biological systems (species, tissue, cell type).
Time-scaleUpon DHT binding to the AR, a conformational change that brings the amino (N) and carboxy (C) termini into close proximity occurs with a t1/2 of approximately 3.5 minutes, around 6 minutes later the AR dimerizes as shown in transfected HeLa cells (Schaufele et al., 2005). Addition of 5 nM DHT to the culture medium of ‘AR-resistant’ transfected prostatic cancer cells resulted in a rapid (from 15 minutes, maximal at 30 minutes) nuclear translocation of the AR with minimal residual cytosolic expression (Nightingale et al., 2003). AR and promoter interactions occur within 15 minutes of ligand binding, and RNA polymerase II and coactivator recruitment are then proposed to occur transiently with cycles of approximately 90 minutes (Kang et al., 2002).
Known modulating factors| Modulating Factor (MF) | MF Specification | Effect(s) on the KER | Reference(s) |
|---|---|---|---|
| Age | AR expression changes with aging | Tissue-specific alterations in AR activity with aging | (Supakar et al., 1993; Wu et al., 2009) |
| Genotype | Number of CAG repeats in the first exon of AR | Decreased AR activation with increased number of CAGs | (Chamberlain et al., 1994; Tut et al., 1997) |
| Androgen deficiency syndrome | Low circulating testosterone levels due to primary (testicular) or secondary (pituitary-hypothalamic) hypogonadism | Reduced levels of circulating testosterone, precurser of DHT | (Bhasin et al., 2010) |
| Castration | Removal of testicles | Reduced levels of circulating testosterone, precurser of DHT | (Krotkiewski et al., 1980) |
Androgens have been shown to upregulate and downregulate AR expression as well as 5α-reductase expression, but for 5α-reductase, each isoform in each tissue is differently regulated by androgens and can display sexual dimorphism (Lee and Chang, 2003; Robitaille and Langlois, 2020). The quantitative impact of such adaptive expression changes is unknown.
References
Aggarwal, S., Thareja, S., Verma, A., Bhardwaj, T.R., Kumar, M., 2010. An overview on 5α-reductase inhibitors. Steroids 75, 109–153. https://doi.org/10.1016/j.steroids.2009.10.005
Bennesch, M.A., Picard, D., 2015. Minireview: Tipping the Balance: Ligand-Independent Activation of Steroid Receptors. Mol. Endocrinol. 29, 349–363. https://doi.org/10.1210/ME.2014-1315
Bhasin, S., Cunningham, G.R., Hayes, F.J., Matsumoto, A.M., Snyder, P.J., Swerdloff, R.S., Montori, V.M., 2010. Testosterone Therapy in Men with Androgen Deficiency Syndromes: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 95, 2536–2559. https://doi.org/10.1210/JC.2009-2354
Bologna, M., Muzi, P., Biordi, L., Festuccia, C., Vicentini, C., 1995. Finasteride dose-dependently reduces the proliferation rate of the LnCap human prostatic cancer cell line in vitro. Urology 45, 282–290. https://doi.org/10.1016/0090-4295(95)80019-0
Bowman, C.J., Barlow, N.J., Turner, K.J., Wallace, D.G., Foster, P.M.D., 2003. Effects of in Utero Exposure to Finasteride on Androgen-Dependent Reproductive Development in the Male Rat. Toxicol. Sci. 74, 393–406. https://doi.org/10.1093/TOXSCI/KFG128
Chamberlain, N.L., Driver, E.D., Miesfeld, R.L., 1994. The length and location of CAG trinucleotide repeats in the androgen receptor N-terminal domain affect transactivation function. Nucleic Acids Res. 22, 3181. https://doi.org/10.1093/NAR/22.15.3181
Costa, E.F., Domenice, S., Sircili, M., Inacio, M., Mendonca, B., 2012. DSD due to 5α-reductase 2 deficiency - From diagnosis to long term outcome. Semin. Reprod. Med. 30, 427–431. https://doi.org/10.1055/S-0032-1324727/ID/JR00766-20/BIB
Dalton, J.T., Mukherjee, A., Zhu, Z., Kirkovsky, L., Miller, D.D., 1998. Discovery of nonsteroidal androgens. Biochem. Biophys. Res. Commun. 244, 1–4. https://doi.org/10.1006/bbrc.1998.8209
Davey, R.A., Grossmann, M., 2016. Androgen Receptor Structure, Function and Biology: From Bench to Bedside. Clin. Biochem. Rev. 37, 3–15.
Eder, I.E., Culig, Z., Putz, T., Nessler-Menardi, C., Bartsch, G., Klocker, H., 2001. Molecular Biology of the Androgen Receptor: From Molecular Understanding to the Clinic. Eur. Urol. 40, 241–251. https://doi.org/10.1159/000049782
Grino, P.B., Griffin, J.E., Wilson, J.D., 1990. Testosterone at High Concentrations Interacts with the Human Androgen Receptor Similarly to Dihydrotestosterone. Endocrinology 126, 1165–1172. https://doi.org/10.1210/endo-126-2-1165
Heinlein, C.A., Chang, C., 2002. Androgen Receptor (AR) Coregulators: An Overview. Endocr. Rev. 23, 175–200. https://doi.org/10.1210/EDRV.23.2.0460
Huggins, C., Hodges, C. V., 1941. Studies on prostatic cancer: I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. Cancer Res. 1, 293–297.
Kang, Z., Pirskanen, A., Jänne, O.A., Palvimo, J.J., 2002. Involvement of proteasome in the dynamic assembly of the androgen receptor transcription complex. J. Biol. Chem. 277, 48366–48371. https://doi.org/10.1074/jbc.M209074200
Keller, E.T., Ershler, W.B., Chang, C., 1996. The androgen receptor: a mediator of diverse responses. Front. Biosci. (Landmark Ed) 1, 59–71. https://doi.org/10.2741/A116
Krotkiewski, M., Kral, J.G., Karlsson, J., 1980. Effects of castration and testosterone substitution on body composition and muscle metabolism in rats. Acta Physiol. Scand. 109, 233–237. https://doi.org/10.1111/J.1748-1716.1980.TB06592.X
Lee, D.K., Chang, C., 2003. Expression and Degradation of Androgen Receptor: Mechanism and Clinical Implication. J. Clin. Endocrinol. Metab. 88, 4043–4054. https://doi.org/10.1210/JC.2003-030261
Marks, L.S., 2004. 5Alpha-Reductase: History and Clinical Importance. Rev. Urol. 6 Suppl 9, S11-21.
Nightingale, J., Chaudhary, K.S., Abel, P.D., Stubbs, A.P., Romanska, H.M., Mitchell, S.E., Stamp, G.W.H., Lalani, E.N., 2003. Ligand Activation of the Androgen Receptor Downregulates E-Cadherin-Mediated Cell Adhesion and Promotes Apoptosis of Prostatic Cancer Cells. Neoplasia 5, 347. https://doi.org/10.1016/S1476-5586(03)80028-3
OECD, 2020. Test No. 458: Stably Transfected Human Androgen Receptor Transcriptional Activation Assay for Detection of Androgenic Agonist and Antagonist Activity of Chemicals, OECD Guidelines for the Testing of Chemicals, Section 4. OECD Publishing, Paris. https://doi.org/10.1787/9789264264366-en
Robitaille, J., Langlois, V.S., 2020. Consequences of steroid-5α-reductase deficiency and inhibition in vertebrates. Gen. Comp. Endocrinol. 290. https://doi.org/10.1016/j.ygcen.2020.113400
Schaufele, F., Carbonell, X., Guerbadot, M., Borngraeber, S., Chapman, M.S., Ma, A.A.K., Miner, J.N., Diamond, M.I., 2005. The structural basis of androgen receptor activation: Intramolecular and intermolecular amino-carboxy interactions. Proc. Natl. Acad. Sci. U. S. A. 102, 9802–9807. https://doi.org/10.1073/pnas.0408819102
Schuppe, E.R., Miles, M.C., Fuxjager, M.J., 2020. Evolution of the androgen receptor: Perspectives from human health to dancing birds. Mol. Cell. Endocrinol. 499, 110577. https://doi.org/10.1016/J.MCE.2019.110577
Schwartz, C.L., Christiansen, S., Vinggaard, A.M., Axelstad, M., Hass, U., Svingen, T., 2019. Anogenital distance as a toxicological or clinical marker for fetal androgen action and risk for reproductive disorders. Arch. Toxicol. 93, 253–272. https://doi.org/10.1007/s00204-018-2350-5
Supakar, P.C., Song, C.S., Jung, M.H., Slomczynska, M.A., Kim, J.M., Vellanoweth, R.L., Chatterjee, B., Roy, A.K., 1993. A novel regulatory element associated with age-dependent expression of the rat androgen receptor gene. J. Biol. Chem. 268, 26400–26408. https://doi.org/10.1016/S0021-9258(19)74328-2
Tut, T.G., Ghadessy, F.J., Trifiro, M.A., Pinsky, L., Yong, E.L., 1997. Long Polyglutamine Tracts in the Androgen Receptor Are Associated with Reduced Trans-Activation, Impaired Sperm Production, and Male Infertility. J. Clin. Endocrinol. Metab. 82, 3777–3782. https://doi.org/10.1210/JCEM.82.11.4385
Williams, A.J., Grulke, C.M., Edwards, J., McEachran, A.D., Mansouri, K., Baker, N.C., Patlewicz, G., Shah, I., Wambaugh, J.F., Judson, R.S., Richard, A.M., 2017. The CompTox Chemistry Dashboard: a community data resource for environmental chemistry. J. Cheminform. 9, 61. https://doi.org/10.1186/s13321-017-0247-6
Wu, D., Lin, G., Gore, A.C., 2009. Age-related Changes in Hypothalamic Androgen Receptor and Estrogen Receptor α in Male Rats. J. Comp. Neurol. 512, 688. https://doi.org/10.1002/CNE.21925
Relationship: 2124: Decrease, AR activation leads to Altered, Transcription of genes by the AR
AOPs Referencing Relationship
Evidence Supporting Applicability of this Relationship
| Term | Scientific Term | Evidence | Links |
|---|---|---|---|
| mammals | mammals | High | NCBI |
| Life Stage | Evidence |
|---|---|
| During development and at adulthood | High |
| Sex | Evidence |
|---|---|
| Mixed | High |
This KER is applicable for both sexes, across developmental stages into adulthood, in numerous cells and tissues and across mammalian taxa. It is, however, acknowledged that this KER most likely has a much broader domain of applicability extending to non-mammalian vertebrates. AOP developers are encouraged to add additional relevant knowledge to expand on the applicability to also include other vertebrates.
Key Event Relationship Description
The androgen receptor (AR) is a ligand-dependent nuclear transcription factor that upon activation translocates to the nucleus, dimerizes, and binds androgen response elements (AREs) to modulate transcription of target genes (Lamont and Tindall, 2010, Roy et al. 2001). Decreased activation of the AR affects its transcription factor activity, therefore leading to altered AR-target gene expression. This KER refers to decreased AR activation and altered gene expression occurring in complex systems, such as in vivo and the specific effect on transcription of AR target genes will depend on species, life stage, tissue, cell type etc.
Evidence Supporting this KER
Biological PlausibilityThe biological plausibility for this KER is considered high
The AR is a ligand-activated transcription factor part of the steroid hormone nuclear receptor family. Non-activated AR is found in the cytoplasm as a multiprotein complex with heat-shock proteins, immunophilins and, other chaperones (Roy et al. 2001). Upon activation through ligand binding, the AR dissociates from the protein complex, translocates to the nucleus and homodimerizes. Facilitated by co-regulators, AR can bind to DNA regions containing AREs and initiate transcription of target genes, that thus will be different in e.g. different tissues, life-stages, species etc.
Through mapping of AREs and ChIP sequencing studies, several AR target genes have been identified, mainly studied in prostate cells (Jin, Kim, and Yu 2013). Different co-regulators and ligands lead to altered expression of different sets of genes (Jin et al. 2013; Kanno et al. 2022). Alternative splicing of the AR can lead to different AR variants that also affects which genes are transcribed (Jin et al. 2013).
Apart from this canonical signaling pathway, the AR can suppress gene expression, indirectly regulate miRNA transcription, and have non-genomic effects by rapid activation of second messenger pathways in either presence or absence of a ligand (Jin et al. 2013).
Empirical EvidenceThe empirical evidence for this KER is considered high
In humans, altered gene expression profiling in individuals with androgen insensitivity syndrome (AIS) can provide supporting empirical evidence (Holterhus et al. 2003; Peng et al. 2021). In rodent AR knockout (KO) models, gene expression profiling studies and gene-targeted approaches have provided information on differentially expressed genes in several organ systems including male and female reproductive, endocrine, muscular, cardiovascular and nervous systems (Denolet et al. 2006; Fan et al. 2005; Holterhus et al. 2003; Ikeda et al. 2005; Karlsson et al. 2016; MacLean et al. 2008; Rana et al. 2011; Russell et al. 2012; Shiina et al. 2006; Wang et al. 2006; Welsh et al. 2012; Willems et al. 2010; Yu et al. 2008, 2012; Zhang et al. 2006; Zhou et al. 2011).
Exposure to known antiandrogens has been shown to alter transcriptional profiles, for example of neonatal pig ovaries (Knapczyk-Stwora et al. 2019).
Dose concordance has also been observed for instance in zebrafish embryos; a dose of 50 µg/L of the AR antagonist flutamide resulted in 674 differentially expressed genes at 96 h post fertilization whereas 500 µg/L flutamide resulted in 2871 differentially expressed genes (Ayobahan et al., 2023).
Uncertainties and InconsistenciesAR action has been reported to occur also without ligand binding. However, not much is known about the extent and biological implications of such non-canonical, ligand-independent AR activation (Bennesch and Picard 2015).
Quantitative Understanding of the Linkage
Response-response relationshipThere is not enough data to define a quantitative relationship between AR activation and alteration of AR target gene transcription, and such a relationship will differ between biological systems (species, tissue, cell type, life stage etc).
Time-scaleAR and promoter interactions occur within 15 minutes of ligand binding, RNA polymerase II and coactivator recruitment are proposed to occur transiently with cycles of approximately 90 minutes in LNCaP cells (Kang et al. 2002). RNA polymerase II elongation rates in mammalian cells have been shown to range between 1.3 and 4.3 kb/min (Maiuri et al. 2011). Therefore, depending on the cell type and the half-life of the AR target gene transcripts, changes are to be expected within hours.
Known modulating factors| Modulating Factor (MF) | MF Specification | Effect(s) on the KER | Reference(s) |
|---|---|---|---|
| Age | AR expression in aging male rats | Tissue-specific alterations in AR activity with aging | (Supakar et al. 1993; Wu, Lin, and Gore 2009) |
| Genotype | Number of CAG repeats in the first exon of AR | Decreased AR activation with increased number of CAGs |
(Tut et al. 1997; Chamberlain et al. 1994) |
AR has been hypothesized to auto-regulate its mRNA and protein levels (Mora and Mahesh 1999).
References
Ayobahan, S. U., Alvincz, J., Reinwald, H., Strompen, J., Salinas, G., Schäfers, C., et al. (2023). Comprehensive identification of gene expression fingerprints and biomarkers of sexual endocrine disruption in zebrafish embryo. Ecotoxicol. Environ. Saf. 250, 114514. doi:10.1016/J.ECOENV.2023.114514.
Bennesch, Marcela A., and Didier Picard. 2015. “Minireview: Tipping the Balance: Ligand-Independent Activation of Steroid Receptors.” Molecular Endocrinology 29(3):349–63.
Chamberlain, Nancy L., Erika D. Driverand, and Roger L. Miesfeldi. 1994. The Length and Location of CAG Trinucleotide Repeats in the Androgen Receptor N-Terminal Domain Affect Transactivation Function. Vol. 22.
Denolet, Evi, Karel De Gendt, Joke Allemeersch, Kristof Engelen, Kathleen Marchal, Paul Van Hummelen, Karen A. L. Tan, Richard M. Sharpe, Philippa T. K. Saunders, Johannes V. Swinnen, and Guido Verhoeven. 2006. “The Effect of a Sertoli Cell-Selective Knockout of the Androgen Receptor on Testicular Gene Expression in Prepubertal Mice.” Molecular Endocrinology 20(2):321–34. doi: 10.1210/me.2005-0113.
Fan, Wuqiang, Toshihiko Yanase, Masatoshi Nomura, Taijiro Okabe, Kiminobu Goto, Takashi Sato, Hirotaka Kawano, Shigeaki Kato, and Hajime Nawata. 2005. Androgen Receptor Null Male Mice Develop Late-Onset Obesity Caused by Decreased Energy Expenditure and Lipolytic Activity but Show Normal Insulin Sensitivity With High Adiponectin Secretion. Vol. 54.
Holterhus, Paul-Martin, Olaf Hiort, Janos Demeter, Patrick O. Brown, and James D. Brooks. 2003. Differential Gene-Expression Patterns in Genital Fibroblasts of Normal Males and 46,XY Females with Androgen Insensitivity Syndrome: Evidence for Early Programming Involving the Androgen Receptor. Vol. 4.
Ikeda, Yasumasa, Ken Ichi Aihara, Takashi Sato, Masashi Akaike, Masanori Yoshizumi, Yuki Suzaki, Yuki Izawa, Mitsunori Fujimura, Shunji Hashizume, Midori Kato, Shusuke Yagi, Toshiaki Tamaki, Hirotaka Kawano, Takahiro Matsumoto, Hiroyuki Azuma, Shigeaki Kato, and Toshio Matsumoto. 2005. “Androgen Receptor Gene Knockout Male Mice Exhibit Impaired Cardiac Growth and Exacerbation of Angiotensin II-Induced Cardiac Fibrosis.” Journal of Biological Chemistry 280(33):29661–66. doi: 10.1074/jbc.M411694200.
Jin, Hong Jian, Jung Kim, and Jindan Yu. 2013. “Androgen Receptor Genomic Regulation.” Translational Andrology and Urology 2(3):158–77.
Kang, Zhigang, Asta Pirskanen, Olli A. Jänne, and Jorma J. Palvimo. 2002. “Involvement of Proteasome in the Dynamic Assembly of the Androgen Receptor Transcription Complex.” Journal of Biological Chemistry 277(50):48366–71. doi: 10.1074/jbc.M209074200.
Kanno, Yuichiro, Nao Saito, Ryota Saito, Tomohiro Kosuge, Ryota Shizu, Tomofumi Yatsu, Takuomi Hosaka, Kiyomitsu Nemoto, Keisuke Kato, and Kouichi Yoshinari. 2022. “Differential DNA-Binding and Cofactor Recruitment Are Possible Determinants of the Synthetic Steroid YK11-Dependent Gene Expression by Androgen Receptor in Breast Cancer MDA-MB 453 Cells.” Experimental Cell Research 419(2). doi: 10.1016/j.yexcr.2022.113333.
Karlsson, Sara A., Erik Studer, Petronella Kettunen, and Lars Westberg. 2016. “Neural Androgen Receptors Modulate Gene Expression and Social Recognition but Not Social Investigation.” Frontiers in Behavioral Neuroscience 10(MAR). doi: 10.3389/fnbeh.2016.00041.
Knapczyk-Stwora, Katarzyna, Anna Nynca, Renata E. Ciereszko, Lukasz Paukszto, Jan P. Jastrzebski, Elzbieta Czaja, Patrycja Witek, Marek Koziorowski, and Maria Slomczynska. 2019. “Flutamide-Induced Alterations in Transcriptional Profiling of Neonatal Porcine Ovaries.” Journal of Animal Science and Biotechnology 10(1):1–15. doi: 10.1186/s40104-019-0340-y.
Lamont, K. R., and Tindall, D. J. (2010). Androgen Regulation of Gene Expression. Adv. Cancer Res. 107, 137–162. doi:10.1016/S0065-230X(10)07005-3.
MacLean, Helen E., W. S. Maria Chiu, Amanda J. Notini, Anna-Maree Axell, Rachel A. Davey, Julie F. McManus, Cathy Ma, David R. Plant, Gordon S. Lynch, and Jeffrey D. Zajac. 2008. “ Impaired Skeletal Muscle Development and Function in Male, but Not Female, Genomic Androgen Receptor Knockout Mice .” The FASEB Journal 22(8):2676–89. doi: 10.1096/fj.08-105726.
Maiuri, Paolo, Anna Knezevich, Alex De Marco, Davide Mazza, Anna Kula, Jim G. McNally, and Alessandro Marcello. 2011. “Fast Transcription Rates of RNA Polymerase II in Human Cells.” EMBO Reports 12(12):1280–85. doi: 10.1038/embor.2011.196.
Mora, Gloria R., and Virendra B. Mahesh. 1999. Autoregulation of the Androgen Receptor at the Translational Level: Testosterone Induces Accumulation of Androgen Receptor MRNA in the Rat Ventral Prostate Polyribosomes.
Peng, Yajie, Hui Zhu, Bing Han, Yue Xu, Xuemeng Liu, Huaidong Song, and Jie Qiao. 2021. “Identification of Potential Genes in Pathogenesis and Diagnostic Value Analysis of Partial Androgen Insensitivity Syndrome Using Bioinformatics Analysis.” Frontiers in Endocrinology 12. doi: 10.3389/fendo.2021.731107.
Rana, Kesha, Barbara C. Fam, Michele V Clarke, Tammy P. S. Pang, Jeffrey D. Zajac, and Helen E. Maclean. 2011. “Increased Adiposity in DNA Binding-Dependent Androgen Receptor Knockout Male Mice Associated with Decreased Voluntary Activity and Not Insulin Resistance.” Am J Physiol Endocrinol Me-Tab 301:767–78. doi: 10.1152/ajpendo.00584.2010.-In.
Roy, Arun K., Rakesh K. Tyagi, Chung S. Song, Yan Lavrovsky, Soon C. Ahn, Tae Sung Oh, and Bandana Chatterjee. 2001. “Androgen Receptor: Structural Domains and Functional Dynamics after Ligand-Receptor Interaction.” Pp. 44–57 in Annals of the New York Academy of Sciences. Vol. 949. New York Academy of Sciences.
Russell, Patricia K., Michele V. Clarke, Jarrod P. Skinner, Tammy P. S. Pang, Jeffrey D. Zajac, and Rachel A. Davey. 2012. “Identification of Gene Pathways Altered by Deletion of the Androgen Receptor Specifically in Mineralizing Osteoblasts and Osteocytes in Mice.” Journal of Molecular Endocrinology 49(1):1–10. doi: 10.1530/JME-12-0014.
Shiina, Hiroko, Takahiro Matsumoto, Takashi Sato, Katsuhide Igarashi, Junko Miyamoto, Sayuri Takemasa, Matomo Sakari, Ichiro Takada, Takashi Nakamura, Daniel Metzger, Pierre Chambon, Jun Kanno, Hiroyuki Yoshikawa, and Shigeaki Kato. 2006. Premature Ovarian Failure in Androgen Receptor-Deficient Mice. Vol. 103.
Supakar, P. C., C. S. Song, M. H. Jung, M. A. Slomczynska, J. M. Kim, R. L. Vellanoweth, B. Chatterjee, and A. K. Roy. 1993. “A Novel Regulatory Element Associated with Age-Dependent Expression of the Rat Androgen Receptor Gene.” Journal of Biological Chemistry 268(35):26400–408. doi: 10.1016/s0021-9258(19)74328-2.
Tut, Thein G., Farid J. Ghadessy, M. A. Trifiro, L. Pinsky, and E. L. Yong. 1997. Long Polyglutamine Tracts in the Androgen Receptor Are Associated with Reduced Trans-Activation, Impaired Sperm Production, and Male Infertility*. Vol. 82.
Wang, Ruey Sheng, Shuyuan Yeh, Lu Min Chen, Hung Yun Lin, Caixia Zhang, Jing Ni, Cheng Chia Wu, P. Anthony Di Sant’Agnese, Karen L. DeMesy-Bentley, Chii Ruey Tzeng, and Chawnshang Chang. 2006. “Androgen Receptor in Sertoli Cell Is Essential for Germ Cell Nursery and Junctional Complex Formation in Mouse Testes.” Endocrinology 147(12):5624–33. doi: 10.1210/en.2006-0138.
Welsh, M., L. Moffat, K. Belling, L. R. de França, T. M. Segatelli, P. T. K. Saunders, R. M. Sharpe, and L. B. Smith. 2012. “Androgen Receptor Signalling in Peritubular Myoid Cells Is Essential for Normal Differentiation and Function of Adult Leydig Cells.” International Journal of Andrology 35(1):25–40. doi: 10.1111/j.1365-2605.2011.01150.x.
Willems, Ariane, Sergio R. Batlouni, Arantza Esnal, Johannes V. Swinnen, Philippa T. K. Saunders, Richard M. Sharpe, Luiz R. França, Karel de Gendt, and Guido Verhoeven. 2010. “Selective Ablation of the Androgen Receptor in Mouse Sertoli Cells Affects Sertoli Cell Maturation, Barrier Formation and Cytoskeletal Development.” PLoS ONE 5(11). doi: 10.1371/journal.pone.0014168.
Wu, D. I., Grace Lin, and Andrea C. Gore. 2009. “Age-Related Changes in Hypothalamic Androgen Receptor and Estrogen Receptor in Male Rats.” The Journal of Comparative Neurology 512:688–701. doi: 10.1002/cne.21925.
Yu, I. Chen, Hung Yun Lin, Ning Chun Liu, Ruey Shen Wang, Janet D. Sparks, Shuyuan Yeh, and Chawnshang Chang. 2008. “Hyperleptinemia without Obesity in Male Mice Lacking Androgen Receptor in Adipose Tissue.” Endocrinology 149(5):2361–68. doi: 10.1210/en.2007-0516.
Yu, Shengqiang, Chiuan Ren Yeh, Yuanjie Niu, Hong Chiang Chang, Yu Chieh Tsai, Harold L. Moses, Chih Rong Shyr, Chawnshang Chang, and Shuyuan Yeh. 2012. “Altered Prostate Epithelial Development in Mice Lacking the Androgen Receptor in Stromal Fibroblasts.” Prostate 72(4):437–49. doi: 10.1002/pros.21445.
Zhang, Caixia, Shuyuan Yeh, Yen-Ta Chen, Cheng-Chia Wu, Kuang-Hsiang Chuang, Hung-Yun Lin, Ruey-Sheng Wang, Yu-Jia Chang, Chamindrani Mendis-Handagama, Liquan Hu, Henry Lardy, Chawnshang Chang, and † † George. 2006. Oligozoospermia with Normal Fertility in Male Mice Lacking the Androgen Receptor in Testis Peritubular Myoid Cells.
Zhou, Wei, Gensheng Wang, Christopher L. Small, Zhilin Liu, Connie C. Weng, Lizhong Yang, Michael D. Griswold, and Marvin L. Meistrich. 2011. “Erratum: Gene Expression Alterations by Conditional Knockout of Androgen Receptor in Adult Sertoli Cells of Utp14bjsd/Jsd (Jsd) Mice (Biology of Reproduction (2010) 83, (759-766) DOI: 10.1095/Biolreprod.110.085472).” Biology of Reproduction 84(2):400–408.
List of Non Adjacent Key Event Relationships
Relationship: 3348: Decrease, AR activation leads to nipple retention, increased
AOPs Referencing Relationship
| AOP Name | Adjacency | Weight of Evidence | Quantitative Understanding |
|---|---|---|---|
| 5α-reductase inhibition leading to increased nipple retention (NR) in male (rodent) offspring | non-adjacent | High | |
| Androgen receptor (AR) antagonism leading to nipple retention (NR) in male (mammalian) offspring | non-adjacent | High | |
| Decreased testosterone synthesis leading to increased nipple retention (NR) in male (rodent) offspring | non-adjacent | High |
Evidence Supporting Applicability of this Relationship
| Life Stage | Evidence |
|---|---|
| Foetal | High |
| Sex | Evidence |
|---|---|
| Male | High |
The KER is considered directly applicable to rats and mice, in which males normally have no nipples due to high levels of androgens during development, leading to regression of nipple anlagen. The empirical evidence supports the relevance to rats, whereas the relevance in mice is assumed based on knowledge about developmental biology in this species. Applicability may extend to most rodents.
While NR is not directly translatable to humans, it serves as a clear indicator of diminished androgen activity causing disrupted fetal masculinisation and sexual differentiation during development - an effect considered relevant to mammals, humans (Schwartz et al., 2021), and vertebrates more broadly (Ogino et al., 2023). NR is included as a mandatory endpoint in several rodent OECD Test Guidelines (OECD 2025a; OECD 2025b, OECD 2025c) and in OECD GD 151 considered an adverse outcome applicable to the setting of Points of Departure for use in human health risk assessment (OECD, 2013). NR can also be used as an indicator of anti-androgenicity in mammals and vertebrates in the environment due to the conserved nature of the AR and its implication in sexual differentiation across species (Ogino et al., 2023).
Life stage
Programming of nipple/areola regression in males occurs during a short window of sensitivity to androgens in the nipple anlagen during fetal life. This takes place in rats around embryonic days 13-15 (Imperato-McGinley et al., 1986), which is, therefore, the relevant window of exposure. The relevant timing for the investigation of NR is PND12-14 in male rat offspring when the nipples are visible in the female littermates. At this time in development, the nipples/areolas are visible through the skin without excessive fur that may interfere with the investigation (Schwartz et al., 2021). It should be mentioned that though the occurrence of nipples/areolas in male offspring is believed to be relatively stable throughout life, it may be responsive to postnatal changes. Permanent nipple/areola retention is observed in some but not all in utero exposure studies with antiandrogens inducing nipple/areola retention at PND 12-14. Most of the differences between studies seem explainable by the window of exposure, dose levels and methods for investigation used, but the responsiveness of nipple/areola retention to postnatal changes remains to be fully explored (Schwartz et al., 2021).
Sex
Data presented in this KER support that disruption of androgen action during fetal life can lead to increased nipple/areola retention in male rat offspring. Since female mice and rat offspring, in general, have 10 (mice) or 12 (rats) nipples at the relevant time of investigation, increased nipple/areola retention at that time point is not a relevant endpoint for females.
Key Event Relationship Description
This KER links a decrease in androgen receptor (AR) activation during fetal development to increased nipple/areola retention (NR) in male rodent offspring. It should be noted that the upstream Key Event (KE) ‘decrease, androgen receptor activation’ (KE-1614 in AOP Wiki) specifically focuses on decreased activation of the AR in vivo, while most methods that can be used to measure AR activity are carried out in vitro. Indirect information about this KE may, for example, be provided from assays showing in vitro AR antagonism, decreased in vitro or in vivo testosterone production/levels, or decreased in vitro or in vivo dihydrotestosterone (DHT) production/levels.
The KER is not directly applicable to humans as both sexes have two nipples, and there is no known effect of androgens on their development (Schwartz et al., 2021). However, NR is a clear readout of reduced androgen action, fetal masculinization and sexual differentiation during development, which is relevant to humans, mammals (Schwartz et al., 2021), and vertebrates more broadly (Ogino et al., 2023). It is included as a mandatory endpoint in several rodent OECD Test Guidelines (OECD, 2025a, 2025b, 2025c) and, in OECD GD 151, is considered an adverse outcome applicable to the setting of Points of Departure for use in human health risk assessment (OECD, 2013). NR can also be used as an indicator of anti-androgenicity in mammals and vertebrates in the environment due to the conserved nature of the AR and its implication in sexual differentiation across species (Ogino et al., 2023).
Evidence Supporting this KER
Biological PlausibilityThe biological plausibility for this KER is judged to be high based on the following:
- Sexual differentiation happens in fetal life. The testes are developed and start to produce testosterone that is converted in other tissues by the enzyme 5-alpha-reductase to the more potent androgen dihydrotestosterone (DHT). Both hormones bind and activate the nuclear receptor and transcription factor AR, which in turn drives the masculinization of the male fetus (Schwartz et al., 2021; Welsh et al., 2014).
- Fetal masculinization depends on the activation of androgen signalling during a critical time window, the masculinization programming window (MPW), from gestational day (GD) 16-20 in rats, 14.5-16.5 in mice and presumably gestation weeks (GWs) 8-14 in humans (Amato et al., 2022; Welsh et al., 2008).
- The fetal masculinization process involves a range of tissues and organs, including the nipple anlagen in rats and mice. In humans, both sexes have two nipples. In contrast, common laboratory mice and rats are sexually dimorphic, with females having 12 (rats) and 10 (mice) nipples and males generally having none (Mayer et al., 2008; Schwartz et al., 2021). In both male and female mouse embryos, stem cells differentiate into a mammary gland, with nipple anlagen being visible by embryonic day 11.5 (Mayer et al., 2008). In male embryos, the presence of androgen leads the nipple anlagen to regress a few days later (Kratochwil, 1977; Kratochwil & Schwartz, 1976) . The androgen responsiveness in the nipple anlagen is rather short, in mice starting late embryonic day 13, with loss of responsiveness on embryonic day 15 (Imperato-McGinley et al., 1986; Kratochwil, 1977) and thus roughly following the timing of the MPW.
- Nipple formation is inhibited in female mice and rat fetuses exposed to androgens during gestation (Goldman et al., 1976; Greene et al., 1941; Imperato-McGinley et al., 1986).
- Male Tfm-mutant mice, which are insensitive to androgens and believed to be so due to a nonfunctional androgen receptor, present with retained nipples (Kratochwil & Schwartz, 1976).
- Multiple mechanisms of action may potentially lead to nipple retention in male mouse and rat offspring. DHT is the main androgen responsible for nipple/areola regression through interaction with AR in the nipple anlagen (Imperato-McGinley et al., 1986). Inhibition of testosterone synthesis or the conversion of testosterone to DHT, increased metabolism of androgens, or direct interference with AR activation may thus all lead to nipple/areola retention (Imperato-McGinley et al., 1986; Schwartz et al., 2021).
Empirical EvidenceThe empirical support from studies in animals for this KER is judged as high overall.
It should be noted that the KE decreased AR activation (KE 1614 in AOP Wiki) specifically focuses on decreased activation of the AR in vivo, with no methods currently available to measure this. Examples of assays that provide indirect information about KE 1614 are described in upstream MIE/KEs.
The empirical evidence for this KER from animal studies in vivo is based on studies using six different substances that result in decreased AR activation by different mechanisms. Flutamide, procymidone and vinclozolin bind to the AR and inhibit the receptor activity and thereby act as AR antagonists, see MIE 26. Finasteride inhibits the 5-alpha-reductase enzyme that converts testosterone to DHT, see MIE 1617. DEHP and DBP exposure during prenatal development in rats results in reduced fetal testosterone levels, see KE-2298 and KE1690. (MIE 26, MIE 1617 and KE 1690 can be found in AOP-Wiki).
The evidence for the upstream KE is mainly based on data from in vitro assays (AR antagonism or 5-alpha-reductase inhibition in vitro), whereas the evidence for the downstream KE is based on in vivo studies, and there is generally no evidence for both KEs from the same study. However, decreased testosterone levels can be measured in vivo, and (Howdeshell et al., 2007; Martino-Andrade et al., 2009) measured the effect of developmental phthalate exposure on both testosterone levels and nipple/areola retention (see the section about “Dose concordance”).
The empirical evidence for the six substances is summarised in Table 3.
Table 3. Summary of empirical evidence for decreased androgen receptor activation, leading to decreased nipple/areola retention. References for the studies supporting the empirical evidence are found in the section “Evidence for decreased AR activation (KE 1614) by flutamide, procymidone, and vinclozolin, finasteride, DEHP and DBP” and in Table 4 in Appendix 2 (6djoma9gmj_KER_3348_Appendix_2_.pdf).
|
Stressor(s) |
Upstream effect |
Downstream effect |
|
Flutamide |
AR antagonism in in vitro assay, receptor binding and transactivation assays
|
Increased NR in males after prenatal exposure in studies in rat
|
|
Procymidone |
AR antagonism in in vitro assay receptor binding and transactivation assays
|
Increased NR in males after prenatal exposure in studies in rat
|
|
Vinclozolin |
AR antagonism in in vitro assay receptor binding and transactivation assays
|
Increased NR in males after prenatal exposure in studies in rat |
|
Finasteride |
Inhibition of 5-alpha-reductase enzyme in in vitro assays
|
Increased NR in males after prenatal exposure in studies in rat
|
|
DEHP |
Reduced production of testosterone in fetal testis measured in ex vivo testis assays, reduced testosterone levels in testis, and reduced fetal plasma or serum testosterone levels
|
Increased NR in males after prenatal exposure in studies in rat
|
|
DBP |
Reduced production of testosterone in fetal testis measured in ex vivo testis assays and reduced testosterone levels in fetal testis |
Increased NR in males after prenatal exposure in studies in rat
|
From Table 3, it can be deduced that fetal exposure to substances known to decrease androgen receptor activation through antagonism of the AR (vinclozolin, procymidone, flutamide), inhibition of testosterone synthesis (DEHP, DBP) or inhibition of the conversion of testosterone to DHT (finasteride), results in increased nipple/areola retention in rat male offspring.
Evidence for decreased AR activation (KE 1614) by flutamide, procymidone, vinclozolin, finasteride, DEHP and DBP.
Flutamide, a pharmaceutical, binds the AR and inhibits its activity, thereby acting as an AR antagonist. It has been used as an antiandrogen for the treatment of prostate cancer and is used as a reference chemical for antiandrogenic activity in the AR transactivation assays in the OECD test guideline No 458 (Goldspiel & Kohler, 1990; Labrie, 1993; OECD, 2023; Simard et al., 1986)
Procymidone and vinclozolin are fungicides that have been shown to be AR antagonists. Procymidone binds to the AR and inhibits the agonist binding, as shown in AR binding assays using rat prostate cytosol (Hosokawa et al., 1993) or AR transfected cells (Ostby et al., 1999). Procymidone also inhibits agonist activated transcription in AR reporter assays (Hass et al., 2012; Kojima et al., 2004; Orton et al., 2011; Ostby et al., 1999; Scholze et al., 2020). Vinclozolin binds to the AR and inhibits the agonist binding, as shown in AR binding assays using rat epididymis cytosol (Kelce & Wilson, 1997) or AR transfected cells (Wong et al., 1995). Vinclozolin also inhibits agonist activated transcription in AR reporter assays (Euling, 2002; Kojima et al., 2004; Molina-Molina et al., 2006; Orton et al., 2011; Scholze et al., 2020; Shimamura et al., 2002; Wong et al., 1995).
Finasteride is a pharmaceutical that inhibits the 5-alpha-reductase enzyme that converts testosterone to DHT. Finasteride is used to treat benign prostatic hypertrophy (Andersson & Russell, 1990; Stoner, 1990; Wood & Rittmaster, 1994).
Prenatal exposure to DEHP in rats has been shown to reduce the production of testosterone in fetal testis measured in ex vivo testis assays, and to reduce testosterone levels in testis and in fetal plasma and serum (Borch et al., 2006; Borch J et al., 2004; Culty et al., 2008; Hannas et al., 2011, 2012; Howdeshell et al., 2007; Klinefelter et al., 2012; Parks, 2000; VO et al., 2009; Wilson et al., 2004, 2007). Conversely, prenatal DEHP exposure did not result in any effects on testosterone levels in the testis at PND1 in one study by Andrade et al. (2006) (Andrade et al., 2006). Similar to DEHP, prenatal exposure to DBP has been shown to reduce the production of testosterone in fetal rat testis measured in ex vivo testis studies (Howdeshell et al., 2007; Wilson et al., 2004) and reduce testosterone levels in the fetal rat testis (Martino-Andrade et al., 2009). The precise underlying mechanism for these effects of DEHP and DPB is presently unknown.
Evidence for increased nipple/areola retention in males (AO-1786) by prenatal exposure to flutamide, procymidone, vinclozolin, finasteride, DEHP and DBP.
All datasets that were used for the weight of evidence assessment were judged as reliable without or with restriction. The majority of datasets assessed showed an increased nipple/areola retention in male offspring after gestational exposure. The conclusion was that the level of confidence was strong for all six substances. The studies are summarised in Table 4 in Appendix 2, 6djoma9gmj_KER_3348_Appendix_2_.pdf
Dose concordance
Dose concordance is challenging to assess for this KER since in vivo AR activity is currently not possible to measure, but can only be inferred indirectly by measures of upstream events. In some studies, fetal (testicular) testosterone levels during, or close to, the fetal masculinization programming window are measured in a subset of animals exposed similarly to those investigated for NR post-natally. Such information may inform on dose concordance if more doses are included.
In a rat in utero exposure study (GD13-21) with DPB and DEHP, testosterone levels in the fetal testes were investigated at GD21, and NR was investigated at PND13 (Martino-Andrade et al., 2009). For DBP, both reduced testosterone levels in fetal testes and NR were observed at 500 mg/kg/d, whereas no effect on NR and only a slight non-significant reduction of testosterone was observed at the lower dose (100 mg/kg/d). For DEHP, a slight but non-significant decrease in testosterone levels in fetal rat testis was observed after exposure to 150 mg/kg/d DEHP, with no effects on nipple/areola retention.
Such data could suggest dose concordance for this part of the KER, although the evidence for this is not strong.
Temporal concordance
Temporal concordance can only be considered from a theoretical perspective since the downstream event, increased NR, is a result of disruption to normal regression of nipple anlagen in male rodents induced during a short window of gestational development (in mice of approximately 2 days), but usually measured at PND12-14 in rats. Earlier than this, the areolae are not yet visible through the skin and later than this, the animals grow fur and need to be shaved for proper examination. This is supported by several of the studies in the empirical evidence, where the test substance was administered during a short period during gestation and nipple retention was observed postnatally.
Based on current knowledge, it is understood that the upstream event – decreased AR activation in vivo – takes place minutes to hours after exposure to an anti-androgenic substance. If a substance decreases AR activation through inhibition of the AR, the upstream event is expected to happen immediately after exposure. If a substance decreases androgen receptor activation through inhibition of testosterone synthesis, the upstream event is expected to happen minutes to hours after the exposure.
Uncertainties and Inconsistencies
For DEHP and DBP, there were some inconsistencies in the empirical evidence, but they could be explained by differences in study designs and uncertainties in measurements (see Appendix 1). Some uncertainty is imposed by the poorly supported dose-concordance. However, the dose-concordance is well supported by the current understanding of biological processes.
Quantitative Understanding of the Linkage
The quantitative understanding of the linkage is low. This is a consequence of it not being possible to measure the upstream and the downstream events in the same study.
Response-response relationshipThe difficulties in extrapolating potency from in vitro to in vivo studies were exemplified by a comparison of the effects of pyrifluquinazon and bisphenol C in vitro and in utero. In vitro, bisphenol C antagonized the androgen receptor with a much higher potency than pyrifluquinazon, but in vivo the potencies were reversed with pyrifluquinazon exposure leading to NR at lower exposure levels than bisphenol C (Gray et al., 2019).
Time-scaleAR activation operates on a time-scale of minutes. The AR is a ligand-activated nuclear receptor and transcription factor. Upon ligand binding a conformational change and subsequent dimerization of the AR takes place within 3-6 minutes (Schaufele et al., 2005). Nuclear translocation (Nightingale et al., 2003) and promoter interactions occur within 15 minutes of ligand binding, and RNA polymerase II and coactivator recruitment are then proposed to occur transiently with cycles of approximately 90 minutes (Kang et al., 2002).
For the downstream event, the time-scale for observing a measurable effect on nipple/areola retention is closer to days and weeks, depending on species. For instance, in mice the nipple anlage are responsive to androgen action at embryonic day 13-15, while a sexual dimorphism of the nipples/areolas can first be observed after birth (Imperato-McGinley et al., 1986) .
Known modulating factorsA well established modulating factor for androgen action is genetic variations in the AR, which decrease the function of the receptor. For example, longer CAG repeat lengths have been associated with decreased AR activation (Chamberlain et al., 1994; Tut et al., 1997). Rat strain is another important modulating factor, with studies showing that the Long-Evans Hooded rat is less sensitive to nipple/areola retention than the Sprague-Dawley rat (Wolf et al., 1999; You et al., 1998)
Known Feedforward/Feedback loops influencing this KERNot relevant for this KER.
References
Amato, C. M., Yao, H. H.-C., & Zhao, F. (2022). One Tool for Many Jobs: Divergent and Conserved Actions of Androgen Signaling in Male Internal Reproductive Tract and External Genitalia. Frontiers in Endocrinology, 13. https://doi.org/10.3389/fendo.2022.910964
Andersson, S., & Russell, D. W. (1990). Structural and biochemical properties of cloned and expressed human and rat steroid 5 alpha-reductases. Proceedings of the National Academy of Sciences, 87(10), 3640–3644. https://doi.org/10.1073/pnas.87.10.3640
Andrade, A. M., Grande, S. W., Talsness, C. E., Grote, K., & Chahoud, I. (2006). Developmental exposure to high and low doses of di-(2-ethylhexyl) phthalate (DEHP); Effects on male rat offspring. NAUNYN-SCHMIEDEBERGS ARCHIVES OF PHARMACOLOGY, 372, 100–101.
Barlow, N. J., McIntyre, B. S., & Foster, P. M. D. (2004). Male reproductive tract lesions at 6, 12, and 18 months of age following in utero exposure to di(n-butyl) phthalate. TOXICOLOGIC PATHOLOGY, 32(1), 79–90. https://doi.org/10.1080/01926230490265894
Borch, J., Axelstad, M., Vinggaard, A. M., & Dalgaard, M. (2006). Diisobutyl phthalate has comparable anti-androgenic effects to di-n-butyl phthalate in fetal rat testis. Toxicology Letters, 163(3), 183–190. https://doi.org/10.1016/j.toxlet.2005.10.020
Borch J, Ladefoged O, Hass U, & Vinggaard AM. (2004). Steroidogenesis in fetal male rats is reduced by DEHP and DINP, but endocrine effects of DEHP are not modulated by DEHA in fetal, prepubertal and adult male rats. Reproductive Toxicology (Elmsford, N.Y.), 18(1), 53–61. https://doi.org/10.1016/j.reprotox.2003.10.011
Chamberlain, N. L., Driver, E. D., & Miesfeld, R. L. (1994). The length and location of CAG trinucleotide repeats in the androgen receptor N-terminal domain affect transactivation function. Nucleic Acids Research, 22(15), 3181–3186. https://doi.org/10.1093/nar/22.15.3181
Christiansen S, Scholze M, Dalgaard M, Vinggaard AM, Axelstad M, Kortenkamp A, & Hass U. (2009). Synergistic disruption of external male sex organ development by a mixture of four antiandrogens. Environmental Health Perspectives, 117(12), 1839–1846. https://doi.org/10.1289/ehp.0900689
Christiansen S, Boberg J, Axelstad M, Dalgaard M, Vinggaard AM, Metzdorff SB, & Hass U. (2010). Low-dose perinatal exposure to di(2-ethylhexyl) phthalate induces anti-androgenic effects in male rats. Reproductive Toxicology (Elmsford, N.Y.), 30(2), 313–321. https://doi.org/10.1016/j.reprotox.2010.04.005
Clewell, R. A., Thomas, A., Willson, G., Creasy, D. M., & Andersen, M. E. (2013). A dose response study to assess effects after dietary administration of diisononyl phthalate (DINP) in gestation and lactation on male rat sexual development. REPRODUCTIVE TOXICOLOGY, 35, 70–80. https://doi.org/10.1016/j.reprotox.2012.07.008
Culty, M., Thuillier, R., Li, W., Wang, Y., Martinez-Arguelles, D. B., Benjamin, C. G., Triantafilou, K. M., Zirkin, B. R., & Papadopoulos, V. (2008). In Utero Exposure to Di-(2-ethylhexyl) Phthalate Exerts Both Short-Term and Long-Lasting Suppressive Effects on Testosterone Production in the Rat1. Biology of Reproduction, 78(6), 1018–1028. https://doi.org/10.1095/biolreprod.107.065649
Euling, S. Y. (2002). Response-Surface Modeling of the Effect of 5alpha-Dihydrotestosterone and Androgen Receptor Levels on the Response to the Androgen Antagonist Vinclozolin. Toxicological Sciences, 69(2), 332–343. https://doi.org/10.1093/toxsci/69.2.332
Fussell KC, Schneider S, Buesen R, Groeters S, Strauss V, Melching-Kollmuss S, & van Ravenzwaay B. (2015). Investigations of putative reproductive toxicity of low-dose exposures to flutamide in Wistar rats. Archives of Toxicology, 89(12), 2385–2402. https://doi.org/10.1007/s00204-015-1622-6
Goldman AS, Shapiro B, & Neumann F. (1976). Role of testosterone and its metabolites in the differentiation of the mammary gland in rats. Endocrinology, 99(6), 1490–1495. https://doi.org/10.1210/endo-99-6-1490
Goldspiel, B. R., & Kohler, D. R. (1990). Flutamide: An Antiandrogen for Advanced Prostate Cancer. DICP, 24(6), 616–623. https://doi.org/10.1177/106002809002400612
Gray LE Jr, Ostby J, Furr J, Price M, Veeramachaneni DN, & Parks L. (2000). Perinatal exposure to the phthalates DEHP, BBP, and DINP, but not DEP, DMP, or DOTP, alters sexual differentiation of the male rat. Toxicological Sciences : An Official Journal of the Society of Toxicology, 58(2), 350–365. https://doi.org/10.1093/toxsci/58.2.350
Gray LE Jr, Barlow NJ, Howdeshell KL, Ostby JS, Furr JR, & Gray CL. (2009). Transgenerational effects of Di (2-ethylhexyl) phthalate in the male CRL:CD(SD) rat: added value of assessing multiple offspring per litter. Toxicological Sciences : An Official Journal of the Society of Toxicology, 110(2), 411–425. https://doi.org/10.1093/toxsci/kfp109
Gray, L. E., Furr, J. R., Conley, J. M., Lambright, C. S., Evans, N., Cardon, M. C., Wilson, V. S., Foster, P. M., & Hartig, P. C. (2019). A Conflicted Tale of Two Novel AR Antagonists in Vitro and in Vivo: Pyrifluquinazon Versus Bisphenol C. Toxicological Sciences, 168(2), 632–643. https://doi.org/10.1093/toxsci/kfz010
Greene, R. R., Burrill, M. W., & Ivy, A. C. (1941). Experimental intersexuality: The effects of combined estrogens and androgens on the embryonic sexual development of the rat. Journal of Experimental Zoology, 87(2), 211–232. https://doi.org/10.1002/jez.1400870203
Hannas, B. R., Furr, J., Lambright, C. S., Wilson, V. S., Foster, P. M. D., & Gray, L. E. (2011). Dipentyl Phthalate Dosing during Sexual Differentiation Disrupts Fetal Testis Function and Postnatal Development of the Male Sprague-Dawley Rat with Greater Relative Potency than Other Phthalates. Toxicological Sciences, 120(1), 184–193. https://doi.org/10.1093/toxsci/kfq386
Hannas, B. R., Lambright, C. S., Furr, J., Evans, N., Foster, P. M. D., Gray, E. L., & Wilson, V. S. (2012). Genomic Biomarkers of Phthalate-Induced Male Reproductive Developmental Toxicity: A Targeted RT-PCR Array Approach for Defining Relative Potency. Toxicological Sciences, 125(2), 544–557. https://doi.org/10.1093/toxsci/kfr315
Hass U, Scholze M, Christiansen S, Dalgaard M, Vinggaard AM, Axelstad M, Metzdorff SB, & Kortenkamp A. (2007). Combined exposure to anti-androgens exacerbates disruption of sexual differentiation in the rat. Environmental Health Perspectives, 115, 122–128. https://doi.org/10.1289/ehp.9360
Hass U, Boberg J, Christiansen S, Jacobsen PR, Vinggaard AM, Taxvig C, Poulsen ME, Herrmann SS, Jensen BH, Petersen A, Clemmensen LH, & Axelstad M. (2012). Adverse effects on sexual development in rat offspring after low dose exposure to a mixture of endocrine disrupting pesticides. Reproductive Toxicology (Elmsford, N.Y.), 34(2), 261–274. https://doi.org/10.1016/j.reprotox.2012.05.090
Hellwig J, van Ravenzwaay B, Mayer M, & Gembardt C. (2000). Pre- and postnatal oral toxicity of vinclozolin in Wistar and Long-Evans rats. Regulatory Toxicology and Pharmacology : RTP, 32(1), 42–50. https://doi.org/10.1006/rtph.2000.1400
Holmer, M. L., Zilliacus, J., Draskau, M. K., Hlisníková, H., Beronius, A., & Svingen, T. (2024). Methodology for developing data-rich Key Event Relationships for Adverse Outcome Pathways exemplified by linking decreased androgen receptor activity with decreased anogenital distance. Reproductive Toxicology, 128, 108662. https://doi.org/10.1016/j.reprotox.2024.108662
Hosokawa S, Murakami M, Ineyama M, Yamada T, Yoshitake A, Yamada H, Miyamoto J. The affinity of procymidone to androgen receptor in rats and mice. J Toxicol Sci. 1993 May;18(2):83-93. doi: 10.2131/jts.18.83. PMID: 8331696.
Howdeshell, K. L., Furr, J., Lambright, C. R., Rider, C. V., Wilson, V. S., & Gray, L. E. (2007). Cumulative Effects of Dibutyl Phthalate and Diethylhexyl Phthalate on Male Rat Reproductive Tract Development: Altered Fetal Steroid Hormones and Genes. Toxicological Sciences, 99(1), 190–202. https://doi.org/10.1093/toxsci/kfm069
Imperato-McGinley J, Binienda Z, Gedney J, & Vaughan ED Jr. (1986). Nipple differentiation in fetal male rats treated with an inhibitor of the enzyme 5 alpha-reductase: definition of a selective role for dihydrotestosterone. Endocrinology, 118(1), 132–137. https://doi.org/10.1210/endo-118-1-132
Jarfelt K, Dalgaard M, Hass U, Borch J, Jacobsen H, & Ladefoged O. (2005). Antiandrogenic effects in male rats perinatally exposed to a mixture of di(2-ethylhexyl) phthalate and di(2-ethylhexyl) adipate. Reproductive Toxicology (Elmsford, N.Y.), 19(4), 505–515. https://doi.org/10.1016/j.reprotox.2004.11.005
Kang, H.-Y., Huang, K.-E., Chang, S. Y., Ma, W.-L., Lin, W.-J., & Chang, C. (2002). Differential Modulation of Androgen Receptor-mediated Transactivation by Smad3 and Tumor Suppressor Smad4. Journal of Biological Chemistry, 277(46), 43749–43756. https://doi.org/10.1074/jbc.M205603200
Kelce, W. R., & Wilson, E. M. (1997). Environmental antiandrogens: Developmental effects, molecular mechanisms, and clinical implications. JOURNAL OF MOLECULAR MEDICINE-JMM, 75(3), 198–207. https://doi.org/10.1007/s001090050104
Klinefelter, G. R., Laskey, J. W., Winnik, W. M., Suarez, J. D., Roberts, N. L., Strader, L. F., Riffle, B. W., & Veeramachaneni, D. N. R. (2012). Novel molecular targets associated with testicular dysgenesis induced by gestational exposure to diethylhexyl phthalate in the rat: a role for estradiol. REPRODUCTION, 144(6), 747–761. https://doi.org/10.1530/REP-12-0266
Kojima, H., Katsura, E., Takeuchi, S., Niiyama, K., & Kobayashi, K. (2004). Screening for estrogen and androgen receptor activities in 200 pesticides by in vitro reporter gene assays using Chinese hamster ovary cells. Environmental Health Perspectives, 112(5), 524–531. https://doi.org/10.1289/ehp.6649
Kratochwil, K. (1977). Development and loss of androgen responsiveness in the embryonic rudiment of the mouse mammary gland. Developmental Biology, 61(2), 358–365. https://doi.org/10.1016/0012-1606(77)90305-0
Kratochwil, K., & Schwartz, P. (1976). Tissue interaction in androgen response of embryonic mammary rudiment of mouse: identification of target tissue for testosterone. Proceedings of the National Academy of Sciences, 73(11), 4041–4044. https://doi.org/10.1073/pnas.73.11.4041
Labrie, F. (1993). Mechanism of action and pure antiandrogenic properties of flutamide. Cancer, 72(12 Suppl), 3816–3827. https://doi.org/10.1002/1097-0142(19931215)72:12+<3816::aid-cncr2820721711>3.0.co;2-3
Martino-Andrade AJ, Morais RN, Botelho GG, Muller G, Grande SW, Carpentieri GB, Leão GM, & Dalsenter PR. (2009). Coadministration of active phthalates results in disruption of foetal testicular function in rats. International Journal of Andrology, 32(6), 704–712. https://doi.org/10.1111/j.1365-2605.2008.00939.x
Matsuura I, Saitoh T, Tani E, Wako Y, Iwata H, Toyota N, Ishizuka Y, Namiki M, Hoshino N, Tsuchitani M, Ikeda Y. Evaluation of a two-generation reproduction toxicity study adding endpoints to detect endocrine disrupting activity using lindane. J Toxicol Sci. (2005) Dec;30 Spec No.:135-161. doi: 10.2131/jts.30.s135. PMID: 16641539.
Mayer, J. A., Foley, J., De La Cruz, D., Chuong, C. M., & Widelitz, R. (2008). Conversion of the nipple to hair-bearing epithelia by lowering bone morphogenetic protein pathway activity at the dermal-epidermal interface. The American Journal of Pathology, 173(5), 1339–1348. https://doi.org/10.2353/AJPATH.2008.070920
McIntyre BS, Barlow NJ, & Foster PM. (2001). Androgen-mediated development in male rat offspring exposed to flutamide in utero: permanence and correlation of early postnatal changes in anogenital distance and nipple retention with malformations in androgen-dependent tissues. Toxicological Sciences : An Official Journal of the Society of Toxicology, 62(2), 236–249. https://doi.org/10.1093/toxsci/62.2.236
Molina-Molina JM, Hillenweck A, Jouanin I, Zalko D, Cravedi JP, Fernández MF, Pillon A, Nicolas JC, Olea N, Balaguer P. Steroid receptor profiling of vinclozolin and its primary metabolites. Toxicol Appl Pharmacol. 2006 Oct 1;216(1):44-54. doi: 10.1016/j.taap.2006.04.005. Epub 2006 Jun 5. PMID: 16750840.
Moore RW, Rudy TA, Lin TM, Ko K, & Peterson RE. (2001). Abnormalities of sexual development in male rats with in utero and lactational exposure to the antiandrogenic plasticizer Di(2-ethylhexyl) phthalate. Environmental Health Perspectives, 109(3), 229–237. https://doi.org/10.1289/ehp.01109229
Mylchreest E, Wallace DG, Cattley RC, & Foster PM. (2000). Dose-dependent alterations in androgen-regulated male reproductive development in rats exposed to Di(n-butyl) phthalate during late gestation. Toxicological Sciences : An Official Journal of the Society of Toxicology, 55(1), 143–151. https://doi.org/10.1093/toxsci/55.1.143
Nightingale, J., Chaudhary, K. S., Abel, P. D., Stubbs, A. P., Romanska, H. M., Mitchell, S. E., Stamp, G. W. H., & Lalani, E.-N. (2003). Ligand Activation of the Androgen Receptor Downregulates E-Cadherin-Mediated Cell Adhesion and Promotes Apoptosis of Prostatic Cancer Cells. Neoplasia, 5(4), 347–361. https://doi.org/10.1016/S1476-5586(03)80028-3
OECD (2013), Guidance Document Supporting OECD Test Guideline 443 on the Extended One-Generational Reproductive Toxicity Test, OECD Series on Testing and Assessment, No. 151, OECD Publishing, Paris, ENV/JM/MONO(2013)10
OECD (2023). Test No. 458: Stably Transfected Human Androgen Receptor Transcriptional Activation Assay for Detection of Androgenic Agonist and Antagonist Activity of Chemicals. OECD Guidelines for the Testing of Chemicals, Section 4, OECD Publishing, Paris, https://doi.org/10.1787/9789264264366-en
OECD (2025a), Test No. 443: Extended One-Generation Reproductive Toxicity Study, OECD Guidelines for the Testing of Chemicals, Section 4, OECD Publishing, Paris, https://doi.org/10.1787/9789264185371-en.
OECD (2025b), Test No. 421: Reproduction/Developmental Toxicity Screening Test, OECD Guidelines for the Testing of Chemicals, Section 4, OECD Publishing, Paris, https://doi.org/10.1787/9789264264380-en.
OECD (2025c), Test No. 422: Combined Repeated Dose Toxicity Study with the Reproduction/Developmental Toxicity Screening Test, OECD Guidelines for the Testing of Chemicals, Section 4, OECD Publishing, Paris, https://doi.org/10.1787/9789264264403-en.
Ogino, Y., Ansai, S., Watanabe, E. et al. Evolutionary differentiation of androgen receptor is responsible for sexual characteristic development in a teleost fish. Nat Commun 14, 1428 (2023). https://doi.org/10.1038/s41467-023-37026-6
Orton, F., Rosivatz, E., Scholze, M., & Kortenkamp, A. (2011). Widely Used Pesticides with Previously Unknown Endocrine Activity Revealed as in Vitro Antiandrogens. ENVIRONMENTAL HEALTH PERSPECTIVES, 119(6), 794–800. https://doi.org/10.1289/ehp.1002895
Ostby J, Monosson E, Kelce WR, & Gray LE Jr. (1999). Environmental antiandrogens: low doses of the fungicide vinclozolin alter sexual differentiation of the male rat. Toxicology and Industrial Health, 15(1), 48–64. https://doi.org/10.1177/074823379901500106
Parks, L. G. (2000). The Plasticizer Diethylhexyl Phthalate Induces Malformations by Decreasing Fetal Testosterone Synthesis during Sexual Differentiation in the Male Rat. Toxicological Sciences, 58(2), 339–349. https://doi.org/10.1093/toxsci/58.2.339
Pedersen, E. B., Christiansen, S., & Svingen, T. (2022). AOP key event relationship report: Linking androgen receptor antagonism with nipple retention. In Current Research in Toxicology (Vol. 3). Elsevier B.V. https://doi.org/10.1016/j.crtox.2022.100085
Saillenfait AM, Sabaté JP, & Gallissot F. (2008). Diisobutyl phthalate impairs the androgen-dependent reproductive development of the male rat. Reproductive Toxicology (Elmsford, N.Y.), 26(2), 107–115. https://doi.org/10.1016/j.reprotox.2008.07.006
Saillenfait AM, Sabaté JP, & Gallissot F. (2009). Effects of in utero exposure to di-n-hexyl phthalate on the reproductive development of the male rat. Reproductive Toxicology (Elmsford, N.Y.), 28(4), 468–476. https://doi.org/10.1016/j.reprotox.2009.06.013
Schaufele, F., Carbonell, X., Guerbadot, M., Borngraeber, S., Chapman, M. S., Ma, A. A. K., Miner, J. N., & Diamond, M. I. (2005). The structural basis of androgen receptor activation: Intramolecular and intermolecular amino–carboxy interactions. Proceedings of the National Academy of Sciences, 102(28), 9802–9807. https://doi.org/10.1073/pnas.0408819102
Schneider S, Kaufmann W, Strauss V, & van Ravenzwaay B. (2011). Vinclozolin: a feasibility and sensitivity study of the ILSI-HESI F1-extended one-generation rat reproduction protocol. Regulatory Toxicology and Pharmacology : RTP, 59(1), 91–100. https://doi.org/10.1016/j.yrtph.2010.09.010
Scholze, M., Taxvig, C., Kortenkamp, A., Boberg, J., Christiansen, S., Svingen, T., Lauschke, K., Frandsen, H., Ermler, S., Hermann, S. S., Pedersen, M., Lykkeberg, A. K., Axelstad, M., & Vinggaard, A. M. (2020). Quantitative in Vitro to in Vivo Extrapolation (QIVIVE) for Predicting Reduced Anogenital Distance Produced by Anti-Androgenic Pesticides in a Rodent Model for Male Reproductive Disorders. Environmental Health Perspectives, 128(11), 117005. https://doi.org/10.1289/EHP6774
Schwartz, C. L., Christiansen, S., Hass, U., Ramhøj, L., Axelstad, M., Löbl, N. M., & Svingen, T. (2021). On the Use and Interpretation of Areola/Nipple Retention as a Biomarker for Anti-androgenic Effects in Rat Toxicity Studies. In Frontiers in Toxicology (Vol. 3). Frontiers Media S.A. https://doi.org/10.3389/ftox.2021.730752
Shimamura M, Kodaira K, Kenichi H, Ishimoto Y, Tamura H, Iguchi T. Comparison of antiandrogenic activities of vinclozolin and D,L-camphorquinone in androgen receptor gene transcription assay in vitro and mouse in utero exposure assay in vivo. Toxicology. 2002 May 24;174(2):97-107. doi: 10.1016/s0300-483x(02)00044-6. PMID: 11985887.
Simard, J., Luthy, I., Guay, J., Bélanger, A., & Labrie, F. (1986). Characteristics of interaction of the antiandrogen flutamide with the androgen receptor in various target tissues. Molecular and Cellular Endocrinology, 44(3), 261–270. https://doi.org/10.1016/0303-7207(86)90132-2
Stoner, E. (1990). The clinical development of a 5α-reductase inhibitor, finasteride. The Journal of Steroid Biochemistry and Molecular Biology, 37(3), 375–378. https://doi.org/10.1016/0960-0760(90)90487-6
Test No. 458: Stably Transfected Human Androgen Receptor Transcriptional Activation Assay for Detection of Androgenic Agonist and Antagonist Activity of Chemicals. (2023). OECD. https://doi.org/10.1787/9789264264366-en
Tut, T. G., Ghadessy, F. J., Trifiro, M. A., Pinsky, L., & Yong, E. L. (1997). Long Polyglutamine Tracts in the Androgen Receptor Are Associated with Reduced Trans -Activation, Impaired Sperm Production, and Male Infertility 1. The Journal of Clinical Endocrinology & Metabolism, 82(11), 3777–3782. https://doi.org/10.1210/jcem.82.11.4385
Vo TT, Jung EM, Dang VH, Jung K, Baek J, Choi KC, Jeung EB. Differential effects of flutamide and di-(2-ethylhexyl) phthalate on male reproductive organs in a rat model. J Reprod Dev. 2009 Aug;55(4):400-11. doi: 10.1262/jrd.20220. Epub 2009 Apr 13. PMID: 19367084.
Welsh, M., Saunders, P. T. K., Fisken, M., Scott, H. M., Hutchison, G. R., Smith, L. B., & Sharpe, R. M. (2008). Identification in rats of a programming window for reproductive tract masculinization, disruption of which leads to hypospadias and cryptorchidism. JOURNAL OF CLINICAL INVESTIGATION, 118(4), 1479–1490. https://doi.org/10.1172/JCI34241
Welsh, M., Suzuki, H., & Yamada, G. (2014). The Masculinization Programming Window. In O. Hiort & S. F. Ahmed (Eds.), UNDERSTANDING DIFFERENCES AND DISORDERS OF SEX DEVELOPMENT (DSD) (Vol. 27, pp. 17–27). https://doi.org/10.1159/000363609
Wilson, V. S., Lambright, C., Furr, J., Ostby, J., Wood, C., Held, G., & Gray, L. E. (2004). Phthalate ester-induced gubernacular lesions are associated with reduced insl3 gene expression in the fetal rat testis. Toxicology Letters, 146(3), 207–215. https://doi.org/10.1016/j.toxlet.2003.09.012
Wilson, V. S., Howdeshell, K. L., Lambright, C. S., Furr, J., & Earl Gray, L. (2007). Differential expression of the phthalate syndrome in male SpragueDawley and Wistar rats after in utero DEHP exposure. Toxicology Letters, 170(3), 177–184. https://doi.org/10.1016/j.toxlet.2007.03.004
Wolf C Jr, Lambright C, Mann P, Price M, Cooper RL, Ostby J, Gray LE Jr. Administration of potentially antiandrogenic pesticides (procymidone, linuron, iprodione, chlozolinate, p,p'-DDE, and ketoconazole) and toxic substances (dibutyl- and diethylhexyl phthalate, PCB 169, and ethane dimethane sulphonate) during sexual differentiation produces diverse profiles of reproductive malformations in the male rat. Toxicol Ind Health. 1999 Jan-Mar;15(1-2):94-118. doi: 10.1177/074823379901500109. PMID: 10188194.
Wolf CJ, LeBlanc GA, Ostby JS, & Gray LE Jr. (2000). Characterization of the period of sensitivity of fetal male sexual development to vinclozolin. Toxicological Sciences : An Official Journal of the Society of Toxicology, 55(1), 152–161. https://doi.org/10.1093/toxsci/55.1.152
Wolf CJ, LeBlanc GA, & Gray LE Jr. (2004). Interactive effects of vinclozolin and testosterone propionate on pregnancy and sexual differentiation of the male and female SD rat. Toxicological Sciences : An Official Journal of the Society of Toxicology, 78(1), 135–143. https://pubmed.ncbi.nlm.nih.gov/14736997/
Wong, C. I., Kelce, W. R., Sar, M., & Wilson, E. M. (1995). Androgen Receptor Antagonist versus Agonist Activities on the Fungicide Vinclozolin Relative to Hydroxyflutamide. Nucleic Acids, Protein Synthesis, and Molecular Genetics, 270(34), 19998–20003.
Wood, A. J. J., & Rittmaster, R. S. (1994). Finasteride. New England Journal of Medicine, 330(2), 120–125. https://doi.org/10.1056/NEJM199401133300208
You, L., Casanova, M., Archibeque-Engle, S., Sar, M., Fan, L.-Q., & Heck, A. (1998). Impaired Male Sexual Development in Perinatal Sprague-Dawley and Long-Evans Hooded Rats Exposed in Utero and Lactationally to p,p’-DDE. In TOX1COLOGICAL SCIENCES (Vol. 45). https://academic.oup.com/toxsci/article/45/2/162/1653877