
This AOP is licensed under the BY-SA license. This license allows reusers to distribute, remix, adapt, and build upon the material in any medium or format, so long as attribution is given to the creator. The license allows for commercial use. If you remix, adapt, or build upon the material, you must license the modified material under identical terms.
AOP: 17
Title
Binding of electrophilic chemicals to SH(thiol)-group of proteins and /or to seleno-proteins involved in protection against oxidative stress during brain development leads to impairment of learning and memory
Short name
Graphical Representation
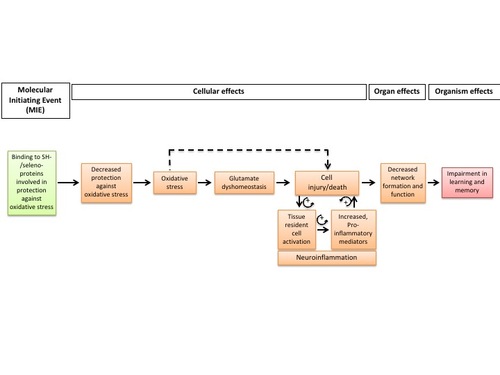
Point of Contact
Contributors
- Florianne Tschudi-Monnet
- Marie-Gabrielle Zurich
Coaches
OECD Information Table
| OECD Project # | OECD Status | Reviewer's Reports | Journal-format Article | OECD iLibrary Published Version |
|---|---|---|---|---|
| 1.13 | WPHA/WNT Endorsed | Scientific Review | iLibrary link |
This AOP was last modified on July 02, 2025 02:28
Revision dates for related pages
| Page | Revision Date/Time |
|---|---|
| Binding, Thiol/seleno-proteins involved in protection against oxidative stress | July 15, 2022 09:18 |
| Increase, Oxidative Stress | February 11, 2026 07:05 |
| Glutamate dyshomeostasis | July 15, 2022 09:45 |
| Increase, Cell injury/death | May 27, 2024 07:23 |
| Neuroinflammation | July 15, 2022 09:54 |
| Decrease of neuronal network function | May 28, 2018 11:36 |
| Impairment, Learning and memory | July 26, 2024 09:54 |
| Tissue resident cell activation | November 15, 2024 10:51 |
| Increased Pro-inflammatory mediators | September 17, 2024 08:35 |
| Decreased protection against oxidative stress | July 15, 2022 09:28 |
| Binding, SH/SeH proteins involved in protection against oxidative stress leads to Protection against oxidative stress, decreased | February 10, 2020 09:40 |
| Protection against oxidative stress, decreased leads to Increase, Oxidative Stress | February 07, 2020 04:27 |
| Increase, Oxidative Stress leads to Glutamate dyshomeostasis | February 06, 2020 11:57 |
| Glutamate dyshomeostasis leads to Cell injury/death | February 06, 2020 11:20 |
| Cell injury/death leads to Neuroinflammation | July 15, 2022 08:26 |
| Cell injury/death leads to Tissue resident cell activation | August 02, 2018 03:02 |
| Neuroinflammation leads to Cell injury/death | November 07, 2019 10:27 |
| Increased pro-inflammatory mediators leads to Cell injury/death | February 12, 2018 04:58 |
| Cell injury/death leads to Neuronal network function, Decreased | February 05, 2020 12:26 |
| Neuronal network function, Decreased leads to Impairment, Learning and memory | July 15, 2022 08:41 |
| Increase, Oxidative Stress leads to Cell injury/death | February 07, 2020 09:32 |
| Methylmercuric(II) chloride | November 29, 2016 18:42 |
| Mercuric chloride | November 29, 2016 18:42 |
| Acrylamide | November 08, 2017 11:15 |
Abstract
This Adverse Outcome Pathway (AOP) describes the linkage between binding to sulfhydryl(SH)-/seleno-proteins involved in protection against oxidative stress and impairment in learning and memory, the Adverse Outcome (AO). Binding to SH-/ seleno-proteins involved in protection against oxidative stress has been defined as the Molecular Initiating Event (MIE). Production, binding and degradation of Reactive Oxygen Radicals (ROS) are tightly regulated, and an imbalance between production and protection may cause oxidative stress, which is common to many toxicity pathways. Oxidative stress may lead to an imbalance in glutamate neurotransmission, which is involved in learning and memory. Oxidative stress may also cause cellular injury and death. During brain development and in particular during the establishment of neuronal connections and networks, such perturbations may lead to functional impairment in learning and memory. Neuroinflammation (Resident cell activation; Increased pro-inflammatory mediators) is triggered early in cell injury cascades and is considered as an exacerbating factor. The weight-of-evidence supporting the relationship between the described key events is based mainly on developmental effects observed after an exposure to the heavy metal, mercury, known for its strong affinity to many SH-/seleno-containing proteins, but in particular to those having anti-oxidant properties, such as glutathione (GSH). The overall assessment of this AOP is considered as strong, based on the biological plausibility, the empirical support and on the essentiality of the Key Events (KEs), which are moderate to strong, since blocking, preventing or attenuating an upstream KE is mitigating the downstream KE. The gap of knowledge is mainly due to limited quantitative evaluations, impeding thus the development of predictive models.
AOP Development Strategy
Context
This AOP was originally started in a workshop report entitled: Adverse Outcome Pathways (AOP) relevant to Neurotoxicity and published in Critical Review in Toxicol: Bal-Price, A., Crofton, K.M., Sachana, M., Shafer, T.J., Behl, M., Forsby, A., Hargreaves, A., Landesmann, B., Lein, P.J., Louisse, J., Monnet-Tschudi, F., Paini, A., Rolaki, A., Schrattenholz, A., Sunol, C., van Thriel, C., Whelan, M., Fritsche, E., 2015. Putative adverse outcome pathways relevant to neurotoxicity. Crit Rev Toxicol 45(1), 83-91.
The process of inflammation is common to many tissues and can be described by several KEs, as proposed in a dedicated workshop (Villeneuve et al., 2018). Brain inflammation called Neuroinflammation can be described by the two common KEs: Tissue resident cell, activation and pro-inflammatory mediators, increased. However, Neuroinflammation is a concept accepted by the regulators and is found in the whole literature describing brain inflammation. Therefore, in accord with the external reviewers, we decided to use the KE Neuroinflammation for building the KERs of this AOP, but we introduced in the list of the KEs the two KEs common to the inflammatory process, as proposed in Villeneuve et al., 2018.
Strategy
Summary of the AOP
Events:
Molecular Initiating Events (MIE)
Key Events (KE)
Adverse Outcomes (AO)
| Type | Event ID | Title | Short name |
|---|
| MIE | 1487 | Binding, Thiol/seleno-proteins involved in protection against oxidative stress | Binding, SH/SeH proteins involved in protection against oxidative stress |
| KE | 1538 | Decreased protection against oxidative stress | Protection against oxidative stress, decreased |
| KE | 1392 | Increase, Oxidative Stress | Increase, Oxidative Stress |
| KE | 1488 | Glutamate dyshomeostasis | Glutamate dyshomeostasis |
| KE | 55 | Increase, Cell injury/death | Cell injury/death |
| KE | 188 | Neuroinflammation | Neuroinflammation |
| KE | 1492 | Tissue resident cell activation | Tissue resident cell activation |
| KE | 1493 | Increased Pro-inflammatory mediators | Increased pro-inflammatory mediators |
| KE | 386 | Decrease of neuronal network function | Neuronal network function, Decreased |
| AO | 341 | Impairment, Learning and memory | Impairment, Learning and memory |
Relationships Between Two Key Events (Including MIEs and AOs)
| Title | Adjacency | Evidence | Quantitative Understanding |
|---|
| Binding, SH/SeH proteins involved in protection against oxidative stress leads to Protection against oxidative stress, decreased | adjacent | Moderate | Moderate |
| Protection against oxidative stress, decreased leads to Increase, Oxidative Stress | adjacent | High | High |
| Increase, Oxidative Stress leads to Glutamate dyshomeostasis | adjacent | Low | Low |
| Glutamate dyshomeostasis leads to Cell injury/death | adjacent | High | Moderate |
| Cell injury/death leads to Neuroinflammation | adjacent | Moderate | |
| Cell injury/death leads to Tissue resident cell activation | adjacent | Moderate | |
| Neuroinflammation leads to Cell injury/death | adjacent | Moderate | |
| Increased pro-inflammatory mediators leads to Cell injury/death | adjacent | Moderate | |
| Cell injury/death leads to Neuronal network function, Decreased | adjacent | Moderate | |
| Neuronal network function, Decreased leads to Impairment, Learning and memory | adjacent | High |
| Increase, Oxidative Stress leads to Cell injury/death | non-adjacent | High | High |
Network View
Prototypical Stressors
Life Stage Applicability
| Life stage | Evidence |
|---|---|
| During brain development |
Taxonomic Applicability
Sex Applicability
| Sex | Evidence |
|---|---|
| Male | |
| Female |
Overall Assessment of the AOP
Experimental and epidemiological evidences indicate that compared to the adult central nervous system (CNS), the developing CNS is generally more susceptible to toxicant exposure (Costa et al., 2004; Grandjean and Landrigan, 2006). Pre-natal and post-natal exposure may have long-term consequences, i.e. not detected immediately at the end of the exposure period. Such effects on visuospatial memory for example have been described on child development in communities with chronic low level mercury exposure (Castoldi et al., 2008a; Debes et al., 2006; Grandjean et al., 2014; Lam et al., 2013).
The aim of this AOP is to capture the KEs and the KERs that occur after binding to thiol- and selenol groups of proteins involved in protection against oxidative stress, the MIE, and impairment in learning and memory, the AO, which is a neurotoxicity marker belonging to the OECD regulatory tool box. The chemical initiators used for the empirical support are methylmercury and mercury chloride, and acrylamide. Data are most extensive for mercury as stressor during development; data for acrylamide are much more limited and restricted to some KEs. Chronic, low-dose prenatal MeHg exposure from maternal consumption of fish has been associated with endpoints of neurotoxicity in children, including poor performance on neurobehavioral tests, particularly on tests of attention, fine-motor function, language, visual-spatial abilities (e.g., drawing), and verbal memory (NRC, 2000). However, it is important to note that some uncertainties remain about the effects of low dose of mercury during brain development (Grandjean et al., 1999). Epidemiological studies in Seychelles on prenatal exposure through fish consumption did not evidenced adverse effects on memory when analyses were performed at 22 and 24 years (Van Wyngaarden et al., 2017), whereas similar experiments made in the Faroe Islands revealed dysfunctions in language, attention and memory at 7 years (Grandjean et al., 1997). And a clear association was observed between mercury cord blood level and memory deficit (Grandjean et al., 1997; Debes et al., 2006). Castoldi and coworkers (2008) proposed that modulating factors, such as diet, nutrition, gender, pattern of exposure and co-exposure could explain the discrepancies of these epidemiological studies. Nevertheless, there are experimental evidences showing that the neurocognitive domain, in particular dentate gyrus, hippocampus and cortex are susceptible to the neurotoxicity of mercury in the developing brain (Sokolowski et al., 2011, 2013; Ceccatelli et al., 2013); therefore, we focus on impairment in learning and memory as the AO. Some –SH- or –SeH-containing proteins involved in protection against oxidative stress have been demonstrated to be inhibited by MeHg either in vitro or in vivo, but a causal relationship has not been established between these inhibitory effects and the final pathological events (Oliveira, 2017). However, the analysis of the essentiality of the KEs and of the weight of evidence for the KERs supports a plausible mechanistic link between the MIE and the AO.
Domain of Applicability
This AOP is mainly focused on the developmental period, although it cannot be excluded that long-term exposure in adult may trigger a similar cascade of KEs leading also to impairment in learning and memory, as observed in neurodegenerative diseases such as Alzheimer's disease (Mutter et al., 2004). While no specific sex differences have been analyzed/described for most KEs, Curtis and coworkers (2010) observed a higher level of TNF-a in hippocampus of male prairie wolf than in female, both treated for 10 weeks with inorganic mercury, in the form of HgCl2; whereas Zhang and coworkers (2013) found a higher neuroinflammatory response associated with altered social behavior in female mice offspring than in male, following gestational exposure to HgCl2. However, after developmental methylmercury exposure, long-lasting behavioral alterations were more prominent in males (Ceccatelli et al., 2013; Castoldi et al., 2008b). These discrepancies may be due to sex differences in kinetics or susceptibility (Vahter et al., 2006).
Essentiality of the Key Events
|
KE |
Defining Question |
High (Strong) |
Moderate |
Low (Weak) |
|
Are downstream KEs and/or the AO prevented if an upstream KE is blocked? |
Direct evidence from specifically designed experimental studies illustrating essentiality for at least one of the important KEs (e.g. stop/reversibility studies, antagonism, KO models, etc.) |
Indirect evidence that sufficient modification of an expected modulating factor attenuates or augments a KE leading to increase in KE down or AO |
No or contradictory experimental evidence on the essentiality of any of the KEs |
|
|
KE1 Decreased protection against oxidative stress |
HIGH |
RATIONALE: The fact that a decrease in anti-oxidant properties causes oxidative stress is well accepted. In addition, experimental evidences of knocking out proteins involved in protection against oxidative stress incresed the susceptibilty to oxidative stress. |
||
|
KE2 Oxidative stress |
HIGH |
RATIONALE: The deleterious consequences of oxidative stress are well accepted in various animal models. Oxygen radical scavengers, such as glutathione, catalase, selenium and cysteine can block the deleterious effects of oxidative stress. |
||
|
KE3 Glutamate dyshomeostasis |
HIGH |
RATIONALE: Glutamate is the main excitatory transmitter, and is involved in memory processes, it is well accepted that perturbation of glutamate homeostasis has deleterious functional consequences. Disruption of glutamate signaling is thought to play a role, at least in part, in the etiology underlying several neurodevelopmental disorders, including memory dysfunction. |
||
|
KE4 Cell Injury/death, increased |
HIGH |
RATIONALE: Cell injury/death is a highly converging node in AOPs. Decrease in synaptic connectivity or cell loss will in turn induce perturbations in the establishment of neuronal connections and trigger inflammatory responses, which through a feedback loop can exacerbate this KE. Therefore, prevention of cell injury/death by anti-oxidant or by inhibitors of NMDA receptors prevents the downstream KEs. |
||
|
KE5 Neuroinflammation KE5' Tissue resident cell activation KE5'' Pro-inflammatory mediators, increased |
MODERATE |
RATIONALE: It is widely accepted in different experimental animal models that the use of minocycline, an antibiotic, which blocks microglial reactivity has protective effects, as have other interferences with any inflammatory mediators. However, we rate the essentiality of this KE as moderate given the complexity of the neuroinflammatory response, having either protective/reparative or aggravating consequences, |
||
|
KE6 Decreased network formation and function |
HIGH |
RATIONALE: Glutamate neurotransmission is an important mechanism underlying memory function (for review: Featherstone, 2010). During brain development, glutamate has also trophic effects, by stimulating BDNF production or through the activation of the different glutamate receptors. The trophic effect of glutamate receptor activation is developmental stage-dependent and may play an important role in determining the selective survival of neurons that made proper connections (Balazs, 2006). |
||
|
AO Impairment of learning and memory |
HIGH |
RATIONALE: Impairment in learning and memory is a converging KE in several AOPs related to brain development. Regarding this AOP and its chemical initiators, it was shown that the neurocognitive domain, in particular dentate gyrus, hippocampus and cortex are susceptible to the neurotoxicity of mercury in the developing brain (Sokolowski et al., 2011, 2013; Ceccatelli et al., 2013). Chronic, low-dose prenatal MeHg exposure from maternal consumption of fish has been associated with endpoints of neurotoxicity in children, including poor performance on neurobehavioral tests, particularly on tests of attention, fine-motor function, language, visual-spatial abilities (e.g., drawing), and verbal memory (NRC, 2000). Prenatal MeHg exposure is associated with childhood memory and learning deficits, particularly visual memory performance in school-aged children (Orenstein, 2014). |
||
Evidence Assessment
Dose-response and temporal concordance of KEs
There is no study where all KEs are measured simultaneously after exposure to several doses, impeding a dose-response and concordance analysis. In one single study (in blue in the table), three downstream KEs were measured following pre-natal exposure to methylmercury. Comparisons of all animal studies show that doses used are ranging from 0.5 - 5 mg/kg; but dose-response was seldom performed. In these studies, the time (pre-natal, post-natal, lactation,...) and duration of exposure are quite diverse and no analysis of brain mercury content was made, so it is not possible to compare doses between studies. Therefore, based on the present data, it is impossible to define whether KEs up occur at lower doses and earlier time points than KEs down.
For in vitro studies, KEs up are often measured after acute exposure to high concentrations.
The following table summarizes concentrations/doses, time, and duration of exposure for the various test systems and KEs.
|
KEs |
In vivo |
In vitro |
|
MIE Binding to SH-/seleno-proteins |
Binding of Hg to thiol groups and to various selenium-containing proteins: Glutathione, thioredoxin reductase, thioredoxin, glutaredoxin, glutathione reductase was measured using purified proteins (Carvahlo et al., 2008, 2011; Wiederhold et al., 2010; Sugiura et al., 1978; Arnold et al., 1986; Han et al., 2001; Qiao et al., 2017) |
|
|
KE1 Decreased protection against oxidative stress |
Cytoplasmic and nuclear TrxR and Cytoplasmic Gpx were reduced in cerebral and cerebellar cortex of 22 days-old offspring (Ruszkiewicz, 2016) Male C57BL/6NJcl mice exposed to methylmercury (1.5 mg/kg/day for 6-weeks) (Fujimura, 2017) Adult male Sprague-Dawley rats exposed to methylmercury (1 mg/kg orally for 6 months) (Joshi, 2014) Zebra fish brain exposed to Hg2+, MeHg 1.8 molar (measured in brain tissue), for 28 days (Branco, 2012) Prenatal and postnatal exposure of mice to 40 ppm of HgCl2 decreased the activity of catalase, thioredoxin reductase, Gpx, superoxide dismutase (Malqui et al., 2017) |
Mouse primary cortical cultures exposed to 5 mM of methylmercury for 24h (Rush, 2012) MeHg inhibits ex vivo rat thioredoxin reductase; IC50 0.158 μM (cerebral) (Wagner et al., 2010) Human neuroblastoma cells (SH-SY5Y)exposed to 1 µM of methylmercury for 6 or 24 h (Branco, 2017; Franco, 2009) |
|
KE2 Oxidative stress |
Male C57BL/6NJcl mice exposed to methylmercury (1.5 mg/kg/day for 6-weeks) (Fujimura, 2017) Adult male Sprague-Dawley rats exposed to methylmercury (1 mg/kg orally for 6 months) (Joshi, 2014) Adult male Sprague-Dawley rats exposed to methylmercury (1 mg/kg orally for 6 months) (Joshi, 2014) Zebra fish brain exposed to Hg2+, MeHg 1.8 molar (measured in brain tissue), for 28 days (Branco, 2012) Prenatal and postnatal exposure of mice to 40 ppm of HgCl2 caused oxidative stress evaluated by increased lipid peroxidation (Malqui et al., 2017) |
Mouse primary cortical cultures exposed to 5 mM of methylmercury for 24h (Rush, 2012) Methylmercury (2-10 µM) in synaptic vesicles isolated from rat brain (with LD50 at 50 µM) (Porciuncula et al., 2003) Human neuroblastoma cells (SH-SY5Y)exposed to 1 µM of methylmercury for 6-24 h (Franco, 2009) |
|
KE3 Glutamate dyshomeostasis |
Rat Young (3-4 weeks) dosed with acrylamide by gavage (5, 15, 30 mg/kg, 5 applications per week during 4 weeks) (Tian, 2018) Microdialysis probe in adult Wistar rats showed that acute exposure to methylmercury (10, 100 mM) induced an increase release of extracellular glutamate (9.8 fold at 10 mM and 2.4 fold at 100 mM). This extracellular glutamate level remained elevated at least 90 min (Juarez et al., 2002) |
Mouse astrocytes, neurons in mono- or co-cultures exposed to methylmercury 1-50 µM for 24h (Morken, 2005) Methylmercury (2-10 µM) in synaptic vesicles isolated from rat brain (with LD50 at 50 µM) (Porciuncula et al., 2003 |
|
KE4 Cell Injury/death, increased |
Rat, perinatal exposure to methylmercury (GD7-PD21, i.e. 35 days) 0.5 mg/kg bw/day in drinking water (Roda et al., 2008) Rat Young (3-4 weeks) exposed to acrylamide by gavage (5, 15, 30 mg/kg, 5 applications per week during 4 weeks) (Tian, 2018) Pregnant rat exposed to methylmercury (1.5 mg/kg orally) from GD5 till parturition (Jacob, 2017) |
Mouse astrocytes, neurons in mono- or co-cultures exposed to methylmercury 1-50 µM for 24h (Morken, 2005) |
|
KE5 Neuroinflammation KE5' Tissue resident cell activation KE5'' Pro-inflammatory mediators, increased |
Rat, perinatal exposure to methylmercury (GD7-PD21, i.e. 35 days) 0.5 mg/kg bw/day in drinking water (Roda et al., 2008) Monkeys, 6,12,18 months oral exposure 50 mg/kg bw (Charleston et al., 1996) |
3D rat brain cell cultures 10 day treatmentHgCl2 10-9-10-6M MeHgCl 10-9-3x10-7M (Monnet-Tschudi et al., 1996; Eskes et al., 2002) |
|
KE6 Decreased network formation and function |
Mice dosed during postnatal week 1-3 with subcutaneous 2-5 mg mercury chloride/kg/once per week (Eddins et al., 2008) Pregnant rat dosed on GD 15 with 8 mg/kg of methylmercury by gavage. Offsprings were tested at day 16, 21 and 60. (Cagiano et al., 1990) Pregnant rat exposed to methylmercury (1.5 mg/kg orally) from GD5 till parturition (Jacob, 2017) |
|
|
AO Impairment of learning and memory |
Mice dosed during postnatal week 1-3 with subcutaneous 2-5 mg mercury chloride/kg/once per week (Eddins et al., 2008) Pregnant rat dosed on GD 15 with 8 mg/kg of methylmercury by gavage. Offsprings were tested at day 16, 21 and 60 (Cagiano et al., 1990) Pregnant rat exposed to methylmercury (1.5 mg/kg orally) from GD5 till parturition (Jacob, 2017) Pregnant mice received 0.5 mg methylmercury/kg/day in drinking water from gestational day 7 until day 7 after delivery. Offspring behavior was monitored at 5-15 and 26-36 weeks of age (Onishchenko et al., 2007) Balb mice exposed to methylmercury in diet (low dose: 1.5 mg/kg; high dose: 4.5 mg/kg) during 11 weeks (6 weeks prior mating, 3 weeks during gestation and 2 weeks post-partum). Offsprings tested at PD 15 showed an accumulation of Hg in brain (0.08 mg/kg for low dose and 0.25 mg/kg for the high dose) (Glover et al., 2009) Prenatal and postnatal exposure of mice to 40 ppm of HgCl2 caused impairment of memory (object recognition, Y maze) Malqui et al., 2017) |
Maternal peripartum hair mercury level was measured to assess prenatal mercury exposure. The concentrations of mercury was found in the range of 0.3-5.1 µg/g, similar to fish-eating population in US. Statistical analyses revealed that each ug/g increase in hair Hg was associated with a decrement in visual memory, learning and verbal memory (Orenstein et al., 2014) Epidemiological studies in the Faroe Islands revealed that mercury exposure through fish consumption (maternal hair conc. 10 ug/g) dysfunctions in memory, language and attention at age 7 (Grandjean et al., 1997; Debes et al., 2006) |
Biological Plausibility and Empirical Support of the KERs
|
KERs |
Defining Question Is there a mechanistic (i.e. structural or functional) relationship between KEup and KEdown consistent with established biological knowledge? |
High (Strong) Extensive understanding of the KER based on extensive previous documentation and broad acceptance |
Moderate The KER is plausible based on analogy to accept biological relationship but scientific understanding is not completely established |
Low (Weak) There is empirical support for a statistical association between KEs but the structural or functional relationship between them is not understood |
|
MIE to KE Decrease protection against oxidative stress |
MODERATE |
RATIONALE: Thiol- and selenol containing proteins, which mainly belong to the anti-oxidant protections, have a high affinity for binding soft metals such as mercury (Farina, 2011). Binding to these thiol/sulfhydryl/SH/SeH groups results in structural modifications affecting the catalytic capacity, and thereby reducing the capacity to neutralize ROS. However, binding to other SH/SeH groups of other proteins not involved in protection against oxidative stress can occur and trigger other neurotoxicity pathways. Alternatively, binding to SH groups of electrophilic compounds may also induce cyto-protective reactions (e.g. via Nrf2). |
||
|
KE Decrease protection against oxidative stress to KE Oxidative stress |
HIGH |
RATIONALE: Oxidative stress is defined as an imbalance in the production of reactive oxygen species (ROS) and antioxidant defenses. Several studies have shown depletion of GSH, the main anti-oxidant, and an increase in oxidative stress following methylmercury or mercury chloride exposures (Meinerz, 2011; Rush, 2012; Agrawal, 2015). Protection against oxidative stress was observed by supplementation with diphenyl selenide (Meinerz, 2011) or by glutathione ester (Rush, 2012). Limited conflicting data. |
||
|
KE Oxidative stress to KE Glutamate (Glu) dyshomeostasis |
LOW |
RATIONALE: Glutamate transport is driven by the Na+ ion gradient, which is dependent on the Na/K ATPase, which, in turn, requires energy. Glutamate enters the cells accompanied by 2 Na+ and one H+. Perturbations of energy metabolism such as mitochondrial dysfunction and increased production of ROS will lead to glutamate dyshomeostasis, due to the indirect coupling of glutamate transporters with ATP level, and to the important role of glutamate transporters in glutamate homeostasis. (Boron and Boulpaep, 2003). Methylmercury was shown to inhibit both the H+-ATPase activity and vesicular glutamate uptake (Porciuncula et al., 2003). As, on one hand, ROS production can interfere with glutamate uptake, and on the other hand, glutamate accumulation leads to excitotoxicity and ROS production, the exact sequence of the KER is difficult to assess. But the fact that both KEs are involved in mercury-induced neurotoxicity is broadly accepted (Farina et al., 2011; Antunes dos Santos et al., 2016; Morris et al., 2017; Kern et al., 2016). |
||
|
KE Glutamate dyshomeostasis to KE Cell injury/death |
HIGH |
RATIONALE: Glutamate dyshomeostasis, in particular excess of glutamate in the synaptic cleft, leads to overactivation of ionotropic glutamate receptors, referred to as excitotoxicity. This, in turn, will cause cell injury/death via ROS production. This KER is also inherent to the developing brain, where glutamate ionotropic receptors are expressed early in various neural cells and when NMDA receptors are expressed in neurons. There is empirical support for all three chemical initiators (mercury, acrylamide, acrolein). In addition, several experiments aiming at blocking glutamate excitotoxicity and the resulting ROS production are protective for cell injury/death. Limited conflicting data. |
||
|
KE Cell injury/death to KE Neuroinflammation |
MODERATE |
RATIONALE: It is widely accepted that cell/neuronal injury and death lead to neuroinflammation (microglial and astrocyte reactivities) in adult brain, and in the developing brain, where neuroinflammation was observed after cell injury/death induced by excitotoxic lesions (Acarin et al., 1997; Dommergues et al., 2003). Empirical support is available for all three chemical initiators (mercury, acrylamide, acrolein). Few experiments, showing a protection when blocking any feature of neuroinflammation have been described. There are some contradicting data showing an absence of neuroinflammatory response despite the occurrence of mercury-induced apotosis and slight behavioral alterations. |
||
|
KE Neuroinflammation to KE Cell injury/death |
MODERATE |
RATIONALE: In vitro co-culture experiments have demonstrated that reactive glial cells (microglia and astrocytes) can kill neurons via the release of pro-inflammatory cytokines, such as TNF-a, IL-1b and IL-6 and/or ROS/RNS (Chao et al., 1995; Brown and Bal-Price, 2003; Kraft and Harry, 2011; Taetzsch and Block, 2013) and that interventions aiming at blocking these inflammatory biomolecules can rescue the neurons (Yadav et al., 2012; Brzozowski et al., 2015). Several reports showed that modulating mercury or acrylamide-induced neuroinflammation was protective for neurons. Because of the complexity of the neuroinflammatory response, that can have neuroprotective or neurodegenerative consequences depending on the duration, local environment or still unknown factors, the rating of this KER was kept as moderate. The vicious cycle between cell injury/death and neuroinflammation is well known and was described in other AOPs. Neuroinflammation could be considered as a modulating factor, but because of the numerous inhibiting experiments, it is considered as an essential KE. Some conflicting data due to the dual role of some inflammatory mediators have been reported. |
||
|
KE Cell injury/death to KE Decreased network formation and function |
HIGH |
RATIONALE: Neuronal network formation and functional crosstalk are established via synaptogenesis. It was shown that under physiological conditions components of the apoptotic machinery in the developing brain regulate synapse formation and neuronal connectivity (Dekkers et al., 2013). The brain’s electrical activity dependence on synapse formation is critical for proper neuronal communication. Glial cells are also involved in the establishment and stabilization of the neuronal network. Extensive experimental support for the adverse effects of mercury on synaptogenesis exist, establishing a strong link between mercury-induced apoptosis and/or neuronal loss and perturbations in a number of neurotransmitter systems (Jacob, 2017; Bridges, 2017) and perturbations of functionality (Falluel-Morel, 2007; Ferraro, 2009; Teixera, 2014; Onishchenko, 2007). Limited protective experiments and conflicting data reported. |
||
|
KE Decreased network formation and function to AO Impairment in learning and memory |
HIGH |
RATIONALE: A review on the Morris water maze (MWM) (Morris, 1981), as an investigative tool of spatial learning and memory in laboratory rats (Vorhees and Williams, 2006) pointed out that perturbed neuronal networks rather than neuronal death per se in certain regions is responsible for the impairment in MWM performance. Functional integrated neural networks that involve the coordination action of different brain regions are consequently important for spatial learning and memory performance (D'Hooge and De Deyn, 2001). Broad empirical support showing mercury-induced effects on learning and memory as consequence of network disruption (Sokolowski et al. 2013; Eddins et al., 2008; Glover et al., 2009). Similar observations were made in humans (Orenstein et al., 2014; Yorifuji et al., 2011). Interestingly, behavioral alterations were detected long time after exposure (delayed effects). Few conflicting data have been reported, but other behavioral deficits, such as alterations in motor activity and increased anxiety suggest that systems other than hippocampus-related learning and memory are also affected. |
||
|
KE oxidative stress to KE Cell injury/death |
HIGH |
RATIONALE: The central nervous system is especially vulnerable to free radical damage since it has a high oxygen consumption rate, an abundant lipid content and reduced levels of antioxidant enzymes (Coyle and Puttfarcken, 1993; Markesbery, 1997). The developing nervous system is particularly vulnerable to chemical insults (Grandjean and Landrigan, 2014). One reason for this higher vulnerability is the incapacity of immature neural cells to cope with oxidative stress by increasing glutathione (GSH) production (Sandström et al., 2017a). Broad empirical support for mercury and acrylamide showing an association between increased ROS production and/or decreased protection against oxidative stress and apoptosis and/or necrosis (Lu et al., 2011; Sarafian et al., 1994; Allam et al., 2011; Lakshmi et al., 2012). Anti-oxidant treatments proved to be protective. Few conflicting data, except a mercury-induced upregulation of GSH level and GR activity as an adaptive mechanism following lactational exposure to methylmercury (10 mg/L in drinking water) associated with motor deficit, suggesting neuronal impairment (Franco et al., 2006). |
||
Known Modulating Factors
| Modulating Factor (MF) | Influence or Outcome | KER(s) involved |
|---|---|---|
Quantitative Understanding
Some quantitative relationships have been described between the upstream early KEs (MIE, oxidative stress, Cell injury/death), although the diversity of test systems and posology (dosing/exposure amount and duration) hampers comparison between studies. It is more difficult to evaluate quantitative relationships between later downstream KEs, such as Neuroinflammation and Decreased Network Function. Neuroinflammation is a complex adaptive mechanism which is not yet completely understood; it can have neuroprotective or neurodegenerative consequences, depending on triggering signals, duration, microenvironment or other unknown influences, which may determine the outcome of the neuroinflammatory process. Decreased network function is currently difficult to quantify because quantitative technologies for mapping and understanding of brain networks (and their plasticity) are still under development.
Optimally, we would like data from a single type of test system showing that exposure to stressor, e.g. mercury, is correlated with changes in all KEs. Such models are emerging, using cells of human origin (Pamies et al., 2016; Sandström et al., 2017b; Fritsche et al., 2017) and/or non-mammalian models, such as zebrafish (Geier et al., 2018; Padilla et al., 2018) and will allow in the future generation of quantitative data which may be used for in silico hazard prediction.
Summary table of Quantitative Evaluations
|
KEs |
Methylmercury (MeHg, CH3Hg) |
|
|
|
|||
|
|
5 µM mouse brain in vitro (Rush, 2012) |
15–30 µM mouse brain, after 40 mg/L in drinking water for 21 days (Glaser, 2013) |
1 µM mouse cerebral cortex ex vivo after oral dosing (Lu et al 2011; conc. from Huang et al 2008) |
17-24, 75-µM (rat cerebral cortex ex vivo after 4w ip dosing) 4w (Xu, 2012; Liu 2013; Feng, 2014) |
|||
|
KE1 Decreased protection against oxidative stress |
GSH reduced 80% of control 24h |
Cortical mitochondrial GPx activity decreased (70% of control), GR increased (170% of control) |
GSH decreased (ca 50% of control) 7 weeks |
Antioxidants NPSH, SOD, GSH-Px decreased (ca 80% and 50% of control) |
|||
|
KE2 Oxidative stress |
ROS increased 120-150% of control 24h |
Cortical mitochondrial TBA-RS increased (ca 140% of control) and complex I, II-III, and IV activity decreased (ca 50% of control). Brain 8-OHdG content increased (ca 400% of control). |
LPO increased (ca 200% of control) 7 weeks |
ROS (DCF) increased (190 and 400% of control at 22,87 μM) |
|||
|
KE3 Glutamate dyshomeostasis |
|
Glutamine synthetase decreased (80 and 50% of control at 24,89 μM) Glutamate content increased (100 and 120% of control at 24,89 µM) Glutamine content decreased (80 and 50% of control at 24,89 μM) |
|||||
|
KE4 Cell Injury/death, increased |
|
Apoptosis-related gene expression: Bcl-2 decreased, ca 50% of control; Bax, Bak, p53, caspase-3,-5,-7 increased, ca 200-350% of control 7 weeks |
Apoptosis increased dose-dependently (300 and 853% of control at 24,89 µM). 8-OHdG expression increased (200 and 450% of control at 24,89 µM) |
||||
|
KE5 Neuroinflammation |
|
||||||
|
KE6 Decreased network formation and function |
|
||||||
|
AO Impairment of learning and memory |
|
||||||
|
KEs |
Mercuric chloride (HgCl2) |
|
|
|
|||
|
|
6 µM rat brain, 1.13 µg Hg/g 6 mo (Agrawal, 2015) |
0.1-100 µM cultured mouse cerebellar granule cells 10 min (Fonfria, 2005) |
|||||
|
KE1 Decreased protection against oxidative stress |
Blood GSH decreased (ca 90% of control) |
|
|||||
|
KE2 Oxidative stress |
|
||||||
|
KE3 Glutamate dyshomeostasis |
Glutamate (3H-aspartate) uptake inhibited (IC50 3.5 uM). Glutamate release stimulated (47% of total endogenous glutamate at 10 µM) |
||||||
|
KE4 Cell Injury/death, increased |
Serum AST increased (ca 140% of control). |
Cell viability (MTT) decreased (ca 10% of control at 10 µM) |
|||||
|
KE5 Neuroinflammation |
|
||||||
|
KE6 Decreased network formation and function |
Brain noradrenaline and dopamine content decreased (ca 30% of control). |
|
|||||
|
AO Impairment of learning and memory |
|
||||||
Considerations for Potential Applications of the AOP (optional)
- Contribution to the network of KEs/AOPs on Developmental Neurotoxicity (DNT)
- Generating quantitative data by measuring all KEs in a single model after repeated/long term exposure to a wide concentration range of the chemical initiators to facilitate the development of computational predictive approaches
References
Acarin, L., B. González, B. Castellano and A. J. Castro (1997). "Quantitative analysis of microglial reaction to a cortical excitotoxic lesion in the early postnatal brain." Exp.Neurol. 147: 410-417.
Agrawal, S., P. Bhatnagar and S. J. Flora (2015). "Changes in tissue oxidative stress, brain biogenic amines and acetylcholinesterase following co-exposure to lead, arsenic and mercury in rats." Food Chem Toxicol 86: 208-216.
Allam, a et al. (2011) ‘Prenatal and perinatal acrylamide disrupts the development of cerebellum in rat: Biochemical and morphological studies.’, Toxicology and industrial health, 27, pp. 291–306. doi: 10.1177/0748233710386412.
Antunes Dos Santos, A., M. Appel Hort, M. Culbreth, C. Lopez-Granero, M. Farina, J. B. Rocha and M. Aschner (2016). "Methylmercury and brain development: A review of recent literature." J Trace Elem Med Biol 38: 99-107.
Arnold AP, Khoon ST, Rabenstein DL (1986) Nuclear magnetic resonance studies of the solution chemistry of metal complexes. 23. Complexation of methylmercury by selenohydryl-containing amino acids and related molecules. Inorganic Chemistry 25 (14), 2433-2437.
Balazs, R. (2006). "Trophic effect of glutamate." Curr Top Med Chem 6(10): 961-968.
Boron, WF and Boulpaep, EL (2005) Medical Physiology. Elsevier. Philadelphia.
Branco, V., J. Canario, J. Lu, A. Holmgren and C. Carvalho (2012). "Mercury and selenium interaction in vivo: effects on thioredoxin reductase and glutathione peroxidase." Free Radic Biol Med 52(4): 781-793.
Branco, V., L. Coppo, S. Sola, J. Lu, C. M. P. Rodrigues, A. Holmgren and C. Carvalho (2017). "Impaired cross-talk between the thioredoxin and glutathione systems is related to ASK-1 mediated apoptosis in neuronal cells exposed to mercury." Redox Biol 13: 278-287.
Bridges, K., Venables, B., Roberts, A., 2017. Effects of dietary methylmercury on the dopaminergic system of adult fathead minnows and their offspring. Environ Toxicol Chem 36, 1077-1084.
Brown, G. C. and A. Bal-Price (2003). "Inflammatory neurodegeneration mediated by nitric oxide, glutamate, and mitochondria." Mol Neurobiol 27(3): 325-355.
Brzozowski, M. J., P. Jenner and S. Rose (2015). "Inhibition of i-NOS but not n-NOS protects rat primary cell cultures against MPP(+)-induced neuronal toxicity." J Neural Transm 122(6): 779-788.
Cagiano, R., et al. (1990). "Evidence that exposure to methyl mercury during gestation induces behavioral and neurochemical changes in offspring of rats." Neurotoxicol Teratol 12(1): 23-28.
Carvalho, C.M. et al. (2008) Inhibition of the human thioredoxin system. A molecular mechanism of mercury toxicity. J Biol Chem 283, 11913-11923.
Carvalho, C.M.L. et al. (2011), Effects of selenite and chelating agents on mammalian thioredoxin reductase inhibited by mercury: Implications for treatment of mercury poisoning(. FASEB Journal, 25 (1), pp. 370-381.
Castoldi, A. F., C. Johansson, N. Onishchenko, T. Coccini, E. Roda, M. Vahter, S. Ceccatelli and L. Manzo (2008a). "Human developmental neurotoxicity of methylmercury: impact of variables and risk modifiers." Regul Toxicol Pharmacol 51(2): 201-214.
Castoldi, A. F., N. Onishchenko, C. Johansson, T. Coccini, E. Roda, M. Vahter, S. Ceccatelli and L. Manzo (2008b). "Neurodevelopmental toxicity of methylmercury: Laboratory animal data and their contribution to human risk assessment." Regul Toxicol Pharmacol 51(2): 215-229.
Ceccatelli, S., R. Bose, K. Edoff, N. Onishchenko and S. Spulber (2013). "Long-lasting neurotoxic effects of exposure to methylmercury during development." J Intern Med 273(5): 490-497.
Charleston, J. S., R. L. Body, R. P. Bolender, N. K. Mottet, M. E. Vahter and T. M. Burbacher (1996). "Changes in the number of astrocytes and microglia in the thalamus of the monkey Macaca fascicularis following long-term subclinical methylmercury exposure." NeuroToxicology 17: 127-138.
Chao, C. C., S. Hu and P. K. Peterson (1995). "Glia, cytokines, and neurotoxicity." Crit.Rev.Neurobiol. 9: 189-205.
Costa LG, Aschner M, Vitalone A, Syversen T, Soldin OP (2004) Developmental neuropathology of environmental agents. Annu Rev Pharmacol Toxicol 44:87-110
Coyle, J. and Puttfarcken, P. (1993) ‘Glutamate Toxicity’, Science, 262, pp. 689–95.
Curtis, J. T., A. N. Hood, Y. Chen, G. P. Cobb and D. R. Wallace (2010). "Chronic metals ingestion by prairie voles produces sex-specific deficits in social behavior: an animal model of autism." Behav Brain Res 213(1): 42-49.
Debes, F., E. Budtz-Jorgensen, P. Weihe, R. F. White and P. Grandjean (2006). "Impact of prenatal methylmercury exposure on neurobehavioral function at age 14 years." Neurotoxicol Teratol 28(5): 536-547.
Dekkers, M.P., Nikoletopoulou, V., Barde, Y.A., 2013. Cell biology in neuroscience: Death of developing neurons: new insights and implications for connectivity. J Cell Biol 203, 385-393.
D'Hooge R, De Deyn PP. (2001). Applications of the Morris water maze in the study of learning and memory. Brain Res Brain Res Rev. 36: 60-90.
Dommergues, M. A., F. Plaisant, C. Verney and P. Gressens (2003). "Early microglial activation following neonatal excitotoxic brain damage in mice: a potential target for neuroprotection." Neuroscience 121(3): 619-628.
Eddins, D., et al. (2008). "Mercury-induced cognitive impairment in metallothionein-1/2 null mice." Neurotoxicol Teratol 30(2): 88-95.
Eskes, C., P. Honegger, L. Juillerat-Jeanneret and F. Monnet-Tschudi (2002). "Microglial reaction induced by noncytotoxic methylmercury treatment leads to neuroprotection via interactions with astrocytes and IL-6 release." Glia 37(1): 43-52.
Falluel-Morel, A., Sokolowski, K., Sisti, H.M., Zhou, X., Shors, T.J., Dicicco-Bloom, E., 2007. Developmental mercury exposure elicits acute hippocampal cell death, reductions in neurogenesis, and severe learning deficits during puberty. J Neurochem 103, 1968-1981.
Farina, M., J. B. Rocha and M. Aschner (2011). "Mechanisms of methylmercury-induced neurotoxicity: evidence from experimental studies." Life Sci 89(15-16): 555-563.
Ferraro, L., Tomasini, M.C., Tanganelli, S., Mazza, R., Coluccia, A., Carratu, M.R., Gaetani, S., Cuomo, V., Antonelli, T., 2009. Developmental exposure to methylmercury elicits early cell death in the cerebral cortex and long-term memory deficits in the rat. Int J Dev Neurosci 27, 165-174.
Featherstone, D. E. (2010). "Intercellular glutamate signaling in the nervous system and beyond." ACS Chem Neurosci 1(1): 4-12.
Feng, S., Xu, Z., Liu, W., Li, Y., Deng, Y., Xu, B., 2014. Preventive effects of dextromethorphan on methylmercury-induced glutamate dyshomeostasis and oxidative damage in rat cerebral cortex. Biol Trace Elem Res 159, 332-345.
Fonfria, E., Vilaro, M.T., Babot, Z., Rodriguez-Farre, E., Sunol, C., 2005. Mercury compounds disrupt neuronal glutamate transport in cultured mouse cerebellar granule cells. J Neurosci Res 79, 545-553.
Franco, J. L. et al. (2006) ‘Cerebellar thiol status and motor deficit after lactational exposure to methylmercury’, Environmental Research, 102(1), pp. 22–28. doi: 10.1016/j.envres.2006.02.003.
Franco, J. L., T. Posser, P. R. Dunkley, P. W. Dickson, J. J. Mattos, R. Martins, A. C. Bainy, M. R. Marques, A. L. Dafre and M. Farina (2009). "Methylmercury neurotoxicity is associated with inhibition of the antioxidant enzyme glutathione peroxidase." Free Radic Biol Med 47(4): 449-457.
Fujimura, M. and F. Usuki (2017). "In situ different antioxidative systems contribute to the site-specific methylmercury neurotoxicity in mice." Toxicology 392: 55-63.
Geier, M.C., Minick, D. J., Truong, L., Tilton, S., Anderson, K.A., Tanguay, R.L., 2018. Systematic developmental neurotoxicity assessment of a representative PAH Superfund mixture using zebrafish. TAAP DNT Special Issue (Submitted).
Glaser, V., B. Moritz, A. Schmitz, A. L. Dafre, E. M. Nazari, Y. M. Rauh Muller, L. Feksa, M. R. Straliottoa, A. F. de Bem, M. Farina, J. B. da Rocha and A. Latini (2013). "Protective effects of diphenyl diselenide in a mouse model of brain toxicity." Chem Biol Interact 206(1): 18-26.
Glover, C. N., et al. (2009). "Methylmercury speciation influences brain gene expression and behavior in gestationally-exposed mice pups." Toxicol Sci 110(2): 389-400.
Grandjean P, Weihe P, White RF, et al. (1997) Cognitive deficit in 7-year-old children with prenatal exposure to methylmercury. Neurotoxicol Teratol 19(6):417-28 doi:10.1016/s0892-0362(97)00097-4
Grandjean P, White RF (1999) Effects of methylmercury exposure on neurodevelopment. JAMA 281:896
Grandjean, P. and P. J. Landrigan (2006). "Developmental neurotoxicity of industrial chemicals." Lancet 368(9553): 2167-2178.
Grandjean, P. and Landrigan, P. J. (2014) ‘Neurobehavioural effects of developmental toxicity’, The Lancet Neurology, 13(3), pp. 330–338. doi: 10.1016/S1474-4422(13)70278-3.
Han S, Zhu M, Yuan Z, Li X (2001) A methylene blue-mediated enzyme electrode for the determination of trace mercury (II), mercury (I), methylmercury, and mercury-glutathione complex. Biosensors & Bioelectronics. 16 : 9-16.
Huang, C.F., Hsu, C.J., Liu, S.H., Lin-Shiau, S.Y., 2008. Neurotoxicological mechanism of methylmercury induced by low-dose and long-term exposure in mice: oxidative stress and down-regulated Na+/K(+)-ATPase involved. Toxicol Lett 176(3), 188-197.
Jacob, S., Thangarajan, S., 2017. Effect of Gestational Intake of Fisetin (3,3',4',7-Tetrahydroxyflavone) on Developmental Methyl Mercury Neurotoxicity in F1 Generation Rats. Biol Trace Elem Res 177, 297-315.
Joshi, D., M. D. Kumar, S. A. Kumar and S. Sangeeta (2014). "Reversal of methylmercury-induced oxidative stress, lipid peroxidation, and DNA damage by the treatment of N-acetyl cysteine: a protective approach." J Environ Pathol Toxicol Oncol 33(2): 167-182.
Juarez BI, Martinez ML, Montante M, Dufour L, Garcia E, Jimenez-Capdeville ME (2002) Methylmercury increases glutamate extracellular levels in frontal cortex of awake rats. Neurotoxicol Teratol 24(6):767-71
Kern, J. K., D. A. Geier, L. K. Sykes, B. E. Haley and M. R. Geier (2016). "The relationship between mercury and autism: A comprehensive review and discussion." J Trace Elem Med Biol 37: 8-24.
Kraft, A. D. and G. J. Harry (2011). "Features of microglia and neuroinflammation relevant to environmental exposure and neurotoxicity." Int J Environ Res Public Health 8(7): 2980-3018.
Lakshmi, D. et al. (2012) ‘Ameliorating effect of fish oil on acrylamide induced oxidative stress and neuronal apoptosis in cerebral cortex’, Neurochemical Research, 37(9), pp. 1859–1867. doi: 10.1007/s11064-012-0794-1.
Lam, H. S., K. M. Kwok, P. H. Chan, H. K. So, A. M. Li, P. C. Ng and T. F. Fok (2013). "Long term neurocognitive impact of low dose prenatal methylmercury exposure in Hong Kong." Environ Int 54: 59-64.
Landa, R. J. (2008). "Diagnosis of autism spectrum disorders in the first 3 years of life." Nat Clin Pract Neurol 4(3): 138-147.
Liu, J. and S. Rozovsky (2013). "Contribution of selenocysteine to the peroxidase activity of selenoprotein S." Biochemistry 52(33): 5514-5516.
Loke, Y. J., A. J. Hannan and J. M. Craig (2015). "The Role of Epigenetic Change in Autism Spectrum Disorders." Front Neurol 6: 107.
Lu, T. H. et al. (2011) ‘Involvement of oxidative stress-mediated ERK1/2 and p38 activation regulated mitochondria-dependent apoptotic signals in methylmercury-induced neuronal cell injury’, Toxicology Letters. Elsevier Ireland Ltd, 204(1), pp. 71–80. doi: 10.1016/j.toxlet.2011.04.013.
Malqui H, Anarghou H, Ouardi FZ, Ouasmi N, Najimi M, Chigr F., Continuous Exposure to Inorganic Mercury Affects Neurobehavioral and Physiological Parameters in Mice., J Mol Neurosci. 2018 Oct;66(2):291-305. doi: 10.1007/s12031-018-1176-1. Epub 2018 Sep
Markesbery, W. R. (1997) ‘Oxidative stress hypothesis in Alzheimer’s disease’, Free Radical Biology and Medicine, 23(1), pp. 134–147. doi: 10.1016/S0891-5849(96)00629-6.
Meinerz, D. F., M. T. de Paula, B. Comparsi, M. U. Silva, A. E. Schmitz, H. C. Braga, P. S. Taube, A. L. Braga, J. B. Rocha, A. L. Dafre, M. Farina, J. L. Franco and T. Posser (2011). "Protective effects of organoselenium compounds against methylmercury-induced oxidative stress in mouse brain mitochondrial-enriched fractions." Braz J Med Biol Res 44(11): 1156-1163.
Monnet-Tschudi, F., M. G. Zurich and P. Honegger (1996). "Comparison of the developmental effects of two mercury compounds on glial cells and neurons in aggregate cultures of rat telencephalon." Brain Res 741(1-2): 52-59.
Morken, T.S., Sonnewald, U., Aschner, M., Syversen, T. (2005). Effects of methylmercury on primary brain cells in mono- and co-culture. Toxicol Sci 87, 169-175.
Morris, G., B. K. Puri, R. E. Frye and M. Maes (2017). "The Putative Role of Environmental Mercury in the Pathogenesis and Pathophysiology of Autism Spectrum Disorders and Subtypes." Mol Neurobiol.
Morris, R.G.M. (1981). Spatial localization does not require the presence of local cues. Learn Motiv. 12: 239-260.
Mutter, J., J. Naumann, C. Sadaghiani, R. Schneider and H. Walach (2004). "Alzheimer disease: mercury as pathogenetic factor and apolipoprotein E as a moderator." Neuro Endocrinol Lett 25(5): 331-339.
National Research Council. 2000. Toxicological Effects of Methylmercury. Washington, DC: The National Academies Press. https://doi.org/10.17226/9899.
Oliveira, C. S., B. C. Piccoli, M. Aschner and J. B. T. Rocha (2017). "Chemical Speciation of Selenium and Mercury as Determinant of Their Neurotoxicity." Adv Neurobiol 18: 53-83.
Onishchenko, N., Tamm, C., Vahter, M., Hokfelt, T., Johnson, J.A., Johnson, D.A., Ceccatelli, S., 2007. Developmental exposure to methylmercury alters learning and induces depression-like behavior in male mice. Toxicol Sci 97, 428-437.
Orenstein, S. T., et al. (2014). "Prenatal organochlorine and methylmercury exposure and memory and learning in school-age children in communities near the New Bedford Harbor Superfund site, Massachusetts." Environ Health Perspect 122(11): 1253-1259.
Padilla, S., Culbreth, M., Deborah, L.H., Olin, J., Jarema, K., Jensen, K., Tennant, A., 2018. Reviewer Selection Summary - Screening for Developmental Neurotoxicity Using Larval Zebrafish: Assessing the Preparation and the Predictive Capability. TAAP DNT Special Issue (Submitted).
Pamies, D., P. Barreras, K. Block, G. Makri, A. Kumar, D. Wiersma, L. Smirnova, C. Zang, J. Bressler, K. M. Christian, G. Harris, G. L. Ming, C. J. Berlinicke, K. Kyro, H. Song, C. A. Pardo, T. Hartung and H. T. Hogberg (2017). "A human brain microphysiological system derived from induced pluripotent stem cells to study neurological diseases and toxicity." ALTEX 34(3): 362-376.
Porciuncula, L. O., J. B. Rocha, R. G. Tavares, G. Ghisleni, M. Reis and D. O. Souza (2003). "Methylmercury inhibits glutamate uptake by synaptic vesicles from rat brain." Neuroreport 14(4): 577-580.
Qiao Y, Huang X, Chen B, He M, Hu B 2017. In vitro study on antagonism mechanism of glutathione, sodium selenite and mercuric chloride. Talanta 171 : 262-269.
Roda, E., T. Coccini, D. Acerbi, A. Castoldi, G. Bernocchi and L. Manzo (2008). "Cerebellum cholinergic muscarinic receptor (subtype-2 and -3) and cytoarchitecture after developmental exposure to methylmercury: an immunohistochemical study in rat." J Chem Neuroanat 35(3): 285-294.
Rush, T., X. Liu, A. B. Nowakowski, D. H. Petering and D. Lobner (2012). "Glutathione-mediated neuroprotection against methylmercury neurotoxicity in cortical culture is dependent on MRP1." Neurotoxicology 33(3): 476-481.
Ruszkiewicz, J. A., A. B. Bowman, M. Farina, J. B. T. Rocha and M. Aschner (2016). "Sex- and structure-specific differences in antioxidant responses to methylmercury during early development." Neurotoxicology 56: 118-126.
Sandstrom J, Broyer A, Zoia D, et al. (2017a) Potential mechanisms of development-dependent adverse effects of the herbicide paraquat in 3D rat brain cell cultures. Neurotoxicology 60:116-124 doi:10.1016/j.neuro.2017.04.010
Sandstrom J, Eggermann E, Charvet I, et al. (2017b) Development and characterization of a human embryonic stem cell-derived 3D neural tissue model for neurotoxicity testing. Toxicol In Vitro 38:124-135 doi:10.1016/j.tiv.2016.10.001
Sarafian, T. A. et al. (1994) ‘Bcl-2 Expression Decreases Methyle Mercury-Induced Free-Radical Generation and Cel Killing in a Neural Cell Line’, Toxicol. Lett., 74(2), pp. 149–155.
Sugiura Y, Tamai Y, Tanaka H. (1978) Selenium protection against mercury toxicity : high binding affinity of methylmercury by selenium-containing ligands in comparison with sulfur-containing ligands. Bioinorg. Chem. 9 :167-180.
Sokolowski, K., A. Falluel-Morel, X. Zhou and E. DiCicco-Bloom (2011). "Methylmercury (MeHg) elicits mitochondrial-dependent apoptosis in developing hippocampus and acts at low exposures." Neurotoxicology 32(5): 535-544.
Sokolowski, K., M. Obiorah, K. Robinson, E. McCandlish, B. Buckley and E. DiCicco-Bloom (2013). "Neural stem cell apoptosis after low-methylmercury exposures in postnatal hippocampus produce persistent cell loss and adolescent memory deficits." Dev Neurobiol 73(12): 936-949.
Taetzsch, T. and M. L. Block (2013). "Pesticides, microglial NOX2, and Parkinson's disease." J Biochem Mol Toxicol 27(2): 137-149.
Teixeira, F.B., Fernandes, R.M., Farias-Junior, P.M., Costa, N.M., Fernandes, L.M., Santana, L.N., Silva-Junior, A.F., Silva, M.C., Maia, C.S., Lima, R.R., 2014. Evaluation of the effects of chronic intoxication with inorganic mercury on memory and motor control in rats. Int J Environ Res Public Health 11, 9171-9185.
Tian, S.M., Ma, Y.X., Shi, J., Lou, T.Y., Liu, S.S., Li, G.Y., 2015. Acrylamide neurotoxicity on the cerebrum of weaning rats. Neural Regen Res 10, 938-943.
Vahter, M., A. Akesson, C. Liden, S. Ceccatelli and M. Berglund (2007). "Gender differences in the disposition and toxicity of metals." Environ Res 104(1): 85-95.
van Wijngaarden E, Thurston SW, Myers GJ, et al. (2017) Methyl mercury exposure and neurodevelopmental outcomes in the Seychelles Child Development Study Main cohort at age 22 and 24years. Neurotoxicol Teratol 59:35-42 doi:10.1016/j.ntt.2016.10.011
Villeneuve DL, Landesmann B, Allavena P, et al. (2018) Representing the Process of Inflammation as Key Events in Adverse Outcome Pathways. Toxicol Sci 163(2):346-352 doi:10.1093/toxsci/kfy047
Vorhees, C.V., Williams, M.T. (2006) Morris waer maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc 1(2): 848-858.
Wagner, C., Sudati, J.H., Nogueira, C.W., Rocha, J.B.T. (2010) In vivo and in vitro inhibition of mice thioredoxin reductase by methylmercury (2010) BioMetals, 23 (6), pp. 1171-1177.
Wiederhold JG, Cramer CJ, Daniel K, Infante I, Bourdon B, Kretzschmar R. (2010) Equilibrium mercury isotope fractionation between dissolved Hg(II) species and thiol-bound Hg. Environ Sci Technol. 44 :4191-7. Doi : 10.1021/es100205t.
Xu, B., Xu, Z.F., Deng, Y., Liu, W., Yang, H.B., Wei, Y.G., 2012. Protective effects of MK-801 on methylmercury-induced neuronal injury in rat cerebral cortex: involvement of oxidative stress and glutamate metabolism dysfunction. Toxicology 300, 112-120.
Yadav, S., S. P. Gupta, G. Srivastava, P. K. Srivastava and M. P. Singh (2012). "Role of secondary mediators in caffeine-mediated neuroprotection in maneb- and paraquat-induced Parkinson's disease phenotype in the mouse." Neurochem Res 37(4): 875-884.
Yorifuji, T., et al. (2011). "Long-term exposure to methylmercury and psychiatric symptoms in residents of Minamata, Japan." Environ Int 37(5): 907-913.
Zhang, Y., V. J. Bolivar and D. A. Lawrence (2013). "Maternal exposure to mercury chloride during pregnancy and lactation affects the immunity and social behavior of offspring." Toxicol Sci 133(1): 101-111.