
This AOP is licensed under the BY-SA license. This license allows reusers to distribute, remix, adapt, and build upon the material in any medium or format, so long as attribution is given to the creator. The license allows for commercial use. If you remix, adapt, or build upon the material, you must license the modified material under identical terms.
AOP: 175
Title
Thyroperoxidase inhibition leading to altered amphibian metamorphosis
Short name
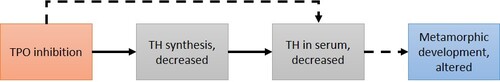
Graphical Representation
Point of Contact
Contributors
- Jonathan Haselman
Coaches
OECD Information Table
| OECD Project # | OECD Status | Reviewer's Reports | Journal-format Article | OECD iLibrary Published Version |
|---|---|---|---|---|
This AOP was last modified on January 11, 2026 16:56
Revision dates for related pages
| Page | Revision Date/Time |
|---|---|
| Thyroperoxidase, Inhibition | November 18, 2025 08:29 |
| Thyroid hormone synthesis, Decreased | November 04, 2022 09:25 |
| Thyroxine (T4) in serum, Decreased | October 10, 2022 08:52 |
| Altered, Amphibian metamorphosis | September 02, 2020 11:19 |
| Thyroperoxidase, Inhibition leads to TH synthesis, Decreased | November 04, 2022 09:27 |
| TH synthesis, Decreased leads to T4 in serum, Decreased | October 10, 2022 08:56 |
| Thyroperoxidase, Inhibition leads to T4 in serum, Decreased | November 18, 2025 08:50 |
| T4 in serum, Decreased leads to Altered, Amphibian metamorphosis | August 25, 2020 16:43 |
| Methimazole | November 29, 2016 18:42 |
| Propylthiouracil | November 29, 2016 18:42 |
| Mercaptobenzothiazole | November 29, 2016 18:42 |
| 2,2',4,4'-Tetrahydroxybenzophenone | November 29, 2016 18:42 |
Abstract
This AOP describes how decreased thyroid hormone (TH) synthesis via chemical inhibition of thyroperoxidase (TPO) causes delayed amphibian metamorphosis, or in extreme cases, arrested development. Amphibian metamorphosis is mediated by TH and successful completion of metamorphosis is generally required for organism survival. TPO is a critical enzyme in the thyroid hormone synthesis pathway that iodinates tyrosyl residues of thyroglobulin and also couples the iodinated tyrosyl residues to form thyroxine (T4). Conversion of T4 to the active hormone, triiodothyronine (T3), is catalyzed by the major activating enzyme, type II iodothyronine deiodinase (see DIO2 AOP 190), located within organs and tissues. T3 then binds to thyroid hormone receptor (THR) along with other cofactors allowing transcriptional gene activation. This T3-mediated gene expression drives the anatomical and physiological changes encompassed by the metamorphic process including limb emergence and development, lung development, gill and tail resorption, gut remodeling, metabolic profile changes in the liver, skin keratinization, etc. The weight of evidence for this AOP is strong, as TPO inhibition has been studied extensively in amphibian model species Xenopus laevis during larval development. Model TPO inhibitors methimazole and 6-propylthiouracil, in addition to other TPO inhibitors such as 2-mercaptobenzothiazole and benzophenone-2, have been tested in this species using several in vivo study designs, some aiming to characterize temporal profiles of glandular hormone levels in addition to serum hormone levels and associated thyroid gland histopathology. These studies lend strong support for essentiality of events proximal to, and including, the MIE. Downstream, the relationship between blood and tissue TH levels is driven by a host of active transporters that have begun to be characterized in mammalian models, whereas THR-mediated gene expression profiles have been studied extensively in the amphibian model. However, nuances of molecular mechanisms at the level of the tissues may lack importance with regard to predicting apical outcomes due to the strong indirect quantitative relationship between circulating hormone levels and metamorphic success.
AOP Development Strategy
Context
Strategy
Summary of the AOP
Events:
Molecular Initiating Events (MIE)
Key Events (KE)
Adverse Outcomes (AO)
| Type | Event ID | Title | Short name |
|---|
| MIE | 279 | Thyroperoxidase, Inhibition | Thyroperoxidase, Inhibition |
| KE | 277 | Thyroid hormone synthesis, Decreased | TH synthesis, Decreased |
| KE | 281 | Thyroxine (T4) in serum, Decreased | T4 in serum, Decreased |
| AO | 1101 | Altered, Amphibian metamorphosis | Altered, Amphibian metamorphosis |
Relationships Between Two Key Events (Including MIEs and AOs)
| Title | Adjacency | Evidence | Quantitative Understanding |
|---|
| Thyroperoxidase, Inhibition leads to TH synthesis, Decreased | adjacent | High | Moderate |
| TH synthesis, Decreased leads to T4 in serum, Decreased | adjacent | High | Moderate |
| Thyroperoxidase, Inhibition leads to T4 in serum, Decreased | non-adjacent | High | High |
| T4 in serum, Decreased leads to Altered, Amphibian metamorphosis | non-adjacent | High | High |
Network View
Prototypical Stressors
Life Stage Applicability
| Life stage | Evidence |
|---|---|
| Development | High |
Taxonomic Applicability
| Term | Scientific Term | Evidence | Link |
|---|---|---|---|
| African clawed frog | Xenopus laevis | High | NCBI |
Sex Applicability
| Sex | Evidence |
|---|---|
| Unspecific | Moderate |
Overall Assessment of the AOP

The overall weight of evidence for this AOP is strong and there is a high level of confidence in the causal linkages between early key events and the adverse outcome. The mechanistic linkages of this pathway have been thoroughly characterized in the model amphbian species, Xenopus laevis, given the critical role of thyroid hormone in controlling amphibian metamorphosis. See attached concordance table.
Domain of Applicability
Chemical: The following chemical classes have been shown to be inhibitors of TPO, but further in vivo characterization of thyroid axis disruption is limited: plant flavonoids (Divi and Doerge, 1996; Doerge and Chang, 2002), benzophenones (Schmutzler et al., 2007), thiocarbamate pesticides (Marinovich et al., 1997) and thioamides (Cooper, 2005). High throughput screening for TPO inhibition has been performed using the in vitro Amplex UltraRed TPO assay developed by Paul et al. (2014). Chemicals in the ToxCast Phase I and II libraries having in vitro activity toward TPO are presented by Paul-Friedman et al. (2016).
Sex: There is no evidence to suggest that TPO inhibition and subsequent thyroid axis disruption during metamorphosis is sexually dimorphic. This AOP is applicable to both males and females.
Life Stage: This AOP is applicable to vertebrate life stages characterized by thyroid hormone-dependent metamorphosis. In the case of Anurans, this AOP applies to larval development.
Taxonomic: Although the weight of evidence assembled for this AOP is based largely on studies using model Anuran species Xenopus laevis (Tietge et al., 2010, 2013; Hornung et al., 2015; Haselman et al., 2020), evidence strongly suggests that this AOP is applicable to all amphibian species (Shi 2000; Dodd and Dodd, 1976). This AOP is also applicable to fish species that undergo thyroid hormone-dependent metamorphosis (Blanton and Specker, 2007; Schreiber and Specker, 1998), but remains tentative due to the paucity of toxicological data demonstrating altered fish metamorphosis due to TPO inhibition.
Essentiality of the Key Events
Although essentiality of key events is typically demonstrated by recovery of normal function following removal of a stressor, metamorphosis is characterized by complex timing of events that, when perturbed, are often not capable of normal recovery due to critical timing errors. For this reason, essentiality of events within this AOP are difficult to evaluate in terms of recovery following removal of a stressor. However, essentiality can be demonstrated in terms of a key event’s propensity to lead to downstream key events based on empirical evidence and biological plausibility.
• Thyroperoxidase, inhibition: [Strong] This enzyme is the primary catalyst for thyroid hormone synthesis. No alternative mechanism has been identified that is capable of synthesizing thyroid hormone. Thyroid hormone is the quintessential molecule necessary for metamorphosis to occur.
• Thyroid hormone synthesis, decreased: [Strong] The process of thyroid hormone synthesis requires iodination of tyrosyl residues of thyroglobulin and subsequent coupling of the iodinated tyrosyl residues to form thyroid hormone. Inhibition of this process by any means will cause decreases in thyroid hormone availability downstream.
• Thyroxine (T4) in serum, decreased: [Strong] The circulatory system is the only means for thyroid hormone delivery from the thyrocytes to peripheral tissues.
• Thyroxine (T4) in tissues, decreased: [Moderate] Triiodothyronine (T3) is the active hormone capable of binding to, and activating, thyroid hormone receptor. There is precedent for local regulation of T3 levels through upregulation of membrane transport proteins and/or cellular deiodinase enzymes acting to increase flux of T4 into the cell and/or increase activation of T4 by deiodination, respectively. These compensatory/local regulatory mechanisms are complex and dynamic making essentiality of this key event more difficult to demonstrate.
• Triiodothyronine (T3) in tissues, decreased: [Strong] T3 is the active hormone capable of binding to thyroid hormone receptor which initiates necessary gene transcription. Although the unliganded receptor plays a role in amphibian development, absence of T3 cannot be augmented by any other process described to date in order to overcome aberrant metamorphic development.
Evidence Assessment
Biological plausibility:
• The critical role of TPO within the thyroid hormone synthesis pathway is well-understood and well-documented in the scientific literature (Divi et al., 1997; Kessler et al., 2008; Ruf and Carayon, 2006; Taurog et al., 1996; Zoeller et al., 2007). Inhibition of this enzyme causes, (1) less iodination of tyrosyl residues of thyroglobulin and (2) less coupling of iodinated tyrosyl residues to form thyroxine; both being the defining mechanisms of TPO. In turn, the inhibition of hormone synthesis leads to decreases in plasma hormone levels, and ultimately leads to less hormone availability to peripheral tissues.
• It is well-established that decreases in thyroid hormone synthesis in the thyroid gland leads to decreased levels of circulating thyroid hormone, as the plasma is the main delivery system for thyroid hormone from the gland to tissues. This relationship has been demonstrated empirically in amphibians following chemical inhibition of TPO by methimazole, 6-propylthiouracil and 2-mercaptobenzothiazole (Tietge 2010, 2013; Haselman et al., 2020).
• Thyroid hormone uptake from plasma into tissues is mediated by a number of active transport proteins exhibiting unique profiles depending on tissue type and timing of development (Hennemann et al., 2001; Visser et al., 2011; Connors et al., 2010). The scientific literature suggests that specific regulation of transporter profiles plays a role in timing of thyroid hormone uptake into specific tissues. However, when thyroid hormone levels in the plasma are deficient, it is highly plausible that tissue levels of thyroid hormone will also be deficient. Studies have shown a strong relationship between plasma and tissue thyroid hormone concentrations, but emphasize the complexity of the relationship due to presumptive local regulation of both active transport and subsequent biochemical modification of thyroid hormone within cells by deiodinase enzymes.
• Thyroid hormone receptor is understood to be highly specific to its native ligand, triiodothyronine (T3). It is well-established that the active hormone, T3, is produced by deiodination of thyroxine (T4), by type I or type II deiodinases, depending on tissue type and timing of development (Gereben et al., 2008a). Based on this understanding, it is highly plausible that when tissue levels of T4 are decreased, this would directly lead to decreased tissue levels of T3. However, there is evidence for local regulation of T3 levels through compensatory expression of deiodinase enzymes (Gereben et al., 2008b), which would ostensibly be capable of T3 homeostasis until T4 levels decrease below a critical threshold.
• Thyroid hormone receptor activation is understood to initiate genetic programs specific to certain tissue types (Wu and Koenig, 2000; Yen et al., 2006) in order to facilitate the metamorphic process (Shi et al., 1996; Brown and Cai, 2007). There is a preponderance of studies that characterize genetic programs in various larval amphibian tissues undergoing thyroid hormone-mediated metamorphosis. It is highly plausible that a decrease in tissue T3 levels leads to decreased activation of the necessary genetic programs that define metamorphosis.
Concordance of dose-response relationships:
• There are two published studies that incorporated a study design addressing dose-response concordance for multiple key events within this AOP (Tietge et al., 2013; Haselman et al., 2020). Aside from these studies, there were several others that evaluated thyroid axis disruption due to putative TPO inhibition, but through evaluations of apical effects and compensatory responses, neither of which lend support to dose-response concordance across key events.
• One example of dose-response concordance for this AOP is demonstrated across multiple studies of TPO inhibitor 2-mercaptobenzothiazole (MBT) (Tietge et al., 2013; Hornung et al., 2015; Paul et al., 2013, 2014; Haselman et al., 2020). In these studies, in vitro TPO enzyme inhibition was tested across a range of MBT concentrations using both rat and porcine enzymes in two different assays employing different assay substrates for either a colorimetric or fluorescence output. In all cases, in vitro results confirmed MBT’s potential to inhibit TPO based on its concentration-response characteristics and relative potency compared to methimazole, a model TPO inhibitor. Without ADME (absorption, distribution, metabolism, excretion) kinetics or direct measures of MBT in the thyroid gland in vivo, IC50 values or effective concentrations from the in vitro assays mean very little in the context of dose-response concordance downstream from this MIE. However, the in vitro assays confirm the potential for MBT to inhibit TPO in vivo. Consistent with MBT’s potential to inhibit TPO, dose-dependent decreases in hormone synthesis within the larval thyroid gland (KE1) was demonstrated by both Tietge et al. (2013) after 7 d of in vivo exposure and Haselman et al. (2020) after 4 d of in vivo exposure to MBT during pro-metamorphosis, and effect concentrations were lower than concentrations necessary to cause subsequent effects in circulating hormone levels (KE2). In a separate study by Tietge et al. (2013), delayed metamorphosis (AO) at 21 d post-NF stage 51 was demonstrated at a similar effect concentration that caused decreased circulating T4 after 7 d post-NF stage 54. Concordant with Tietge et al. (2013), Haselman et al. (2020) demonstrated delayed metamorphosis (AO) as increased time to NF stage 62 in the MBT treatment that showed significantly decreased levels of circulating T4 at 10 d of exposure during pro-metamorphosis (see concordance table for details).
• Haselman et al. (2020) demonstrated in vivo dose-response concordance using model TPO inhibitors methimazole, 6-propylthiouracil and mercaptobenzothiazole. This study was designed specifically to evaluate concordance of both dose-response and temporal responses across KE1, KE2 and the AO using three different TPO inhibitors. Dose-dependent decreases in glandular T4 (KE1) and circulating T4 (KE2) were demonstrated for all three chemicals. Although these biochemical responses showed dose dependence, the AO (evaluated as time to NF stage 62) showed more of a binary response, indicating a tipping point beyond which the organisms could not compensate (see concordance table for details).
Temporal concordance among the key events and adverse effect:
• Tietge et al. (2010) specifically addressed temporal effects of thyroid hormone synthesis inhibition using model TPO inhibitors methimazole and 6-propylthiouracil. However, the study employed a single high effective dose of each chemical to evaluate time-dependent effects of TPO inhibition on early biochemical indicators. The clearest example of temporal concordance for this AOP was demonstrated by the pharmaceutical TPO inhibitor, methimazole. Pro-metamorphic NF54 tadpoles exposed for 2 d exhibited significantly decreased thyroid hormone synthesis (KE1) whereas significantly decreased circulating T4 (KE2) was not observed until 6 d following initiation of exposure. Delayed metamorphosis (AO) was not observed throughout the 8 d exposure period in this study, but was observed in three separate studies following exposure to methimazole at both 14 d and 21 d post-NF51 and 14 d post-NF54, supporting temporal concordance between early KEs and the AO (Degitz et al., 2005; Coady et al., 2010). Temporal concordance between KEs and the AO was also demonstrated with TPO inhibitors 6-propylthiouracil (Tietge et al., 2010; Degitz et al., 2005) and 2-mercaptobenzothiazole (Tietge et al., 2013), which can also be viewed in the attached concordance table.
• Haselman et al. (2020) also specifically addressed temporal effects of thyroid hormone synthesis inhibition using model TPO inhibitors methimazole, 6-propylthiouracil and 2-mercaptobenzothiazole. This study aimed to characterize effects on thyroid biochemistry both temporally and dose-response from exposure to the three chemicals throughout a 10 day period during pro-metamorphosis and link those effects to metamorphic success/failure (AO). Thyroid hormone synthesis in the gland (KE1) was significantly decreased by 2 d of exposure whereas significant decreases in circulating TH did not occur until 7 d of exposure. The authors defined the AO as the time to reach metamorphic climax, so the temporal concordance with the AO is an artifact of the study design. However, based on the developmental staging data, there appears to be temporal concordance with the AO, as the effect on metamorphic rates gains resolution over time and with increasing levels of exposure.
Consistency:
• The collection of TPO inhibition studies in Xenopus laevis demonstrate the consistency of responses in early KEs with associated effects on metamorphosis (AO). Further, these studies also demonstrate consistent compensatory responses to thyroid axis perturbations through thyroid gland histopathology evaluations, which exemplify the underlying feedback mechanisms not captured by KEs in this AOP. These compensatory responses can consistently be observed as early as changes in biochemical profiles can be measured in KE1. Methods to measure these compensatory responses are well-established and more transferrable making them equally acceptable diagnostic indicators for thyroid axis disruption, but generally lend little support for AOP development, and especially quantitative AOP development, as the AOP framework doesn’t currently provide a clearly defined venue for biological feedback mechanisms and compensation. However, the evidence consistently, and strongly, suggests that perturbations of the thyroid axis via TPO inhibition leads to delayed metamorphosis.
Uncertainties, inconsistencies and data gaps:
• There are several areas of uncertainty and one major data gap with regard to this AOP. First, peripheral tissue concentrations of T4 and T3 in amphibians have not yet been measured leaving a data gap between upstream key events and the AO. This is not surprising given the complexities surrounding these analyses including, (1) the low levels of thyroid hormones in other tissues relative to the blood and thyroid gland, (2) small peripheral tissue masses in amphibian larvae, (3) cumbersome extraction methods and (4) expensive instrumental analyses. Although methods are currently being developed to make these measurements, they will likely never become routine, nor will they be entirely necessary to support this AOP due to the strong relationship between circulating thyroid hormone levels (KE2) and the AO. Next, research in the area of amphibian population modeling has emphasized the complexities associated with amphibian populations and how their unique environments influence their ability or inability to persist. Although there are efforts to model amphibian ecology, there are no known active research efforts to link altered metamorphosis to population trajectories, so there is great uncertainty around downstream events subsequent to the AO. Along these lines, much of the weight of evidence for this AOP is based on studies using model amphibian species Xenopus laevis. Therefore, there is uncertainty regarding cross-species sensitivity and/or susceptibility to TPO inhibition leading to altered metamorphosis. Finally, there is uncertainty and/or data gaps around extrapolation from in vitro TPO inhibition data to in vivo effective doses. Extrapolation models of this sort exist for some mammals and fish, but there are no known current efforts to model these relationships in amphibians.
Known Modulating Factors
Quantitative Understanding
Assessment of quantitative understanding of the AOP:
• The amphibian thyroid axis and its critical role in metamorphosis is the hallmark of this AOP. Computational modeling of a normal (and perturbed) Xenopus laevis thyroid axis would require the mathematical characterization of mass balance relationships between iodine and iodinated biomolecules, feedback and feedforward mechanisms, in addition to clearance rates and other parameters to allow a dynamic model to accurately predict key event trends and critical thresholds. Currently, there is enough data and tools that exist to allow development of such a model to predict in vivo effects based on in vitro TPO inhibition screening data. However, this greatly depends on the strength of the relationship between circulating T4 (KE2) and altered metamorphosis (AO), which has been quantiatively demonstrated by Haselman et al. (2020). This quantitative relationship supersedes the need for tissue levels of T4 and T3 (KE3 and KE4, respectively), which currently doesn’t exist for amphibians and are exceedingly complicated/expensive to measure. When considering the dynamic nature of thyroid homeostasis and metamorphosis, this linear AOP is oversimplified and would need to be represented within a larger, more complex systems model. On either side of the MIE, tools exist that can be applied to address exposure and in vitro to in vivo extrapolation of dose and response.
Considerations for Potential Applications of the AOP (optional)
Regulatory testing guidelines (OECD 2009, 2012; US EPA 2009, 2015) rely upon Xenopus laevis metamorphosis as a means to evaluate thyroid disrupting chemicals but primarily characterize apical outcomes relevant to risk assessment (i.e., delayed/arrested metamorphosis). This AOP supports a set of the mechanistic linkages that can cause a regulatory-relavant adverse outcome and supports the notion that biochemical measurements, particularly circulating T4, can be predictive of this apical outcome. The strong linkage between circulating T4 and altered metamorphosis demonstrated by research supporting this AOP is applicable beyond this AOP to any AOP linking KE281 to AO1101.
References
Doerge, D.R. and Chang, H.C., 2002. Inactivation of thyroid peroxidase by soy isoflavones, in vitro and in vivo. Journal of Chromatography B, 777(1), pp.269-279.
Divi, R.L. and Doerge, D.R., 1996. Inhibition of thyroid peroxidase by dietary flavonoids. Chemical research in toxicology, 9(1), pp.16-23.
Blanton, M.L. and Specker, J.L., 2007. The hypothalamic-pituitary-thyroid (HPT) axis in fish and its role in fish development and reproduction. Critical reviews in toxicology, 37(1-2), pp.97-115.
Brown, D.D. and Cai, L., 2007. Amphibian metamorphosis. Developmental biology, 306(1), pp.20-33.
Connors, K.A., Korte, J.J., Anderson, G.W. and Degitz, S.J., 2010. Characterization of thyroid hormone transporter expression during tissue-specific metamorphic events in Xenopus tropicalis. General and comparative endocrinology, 168(1), pp.149-159.
Cooper, D.S., 2005. Antithyroid drugs. New England Journal of Medicine, 352(9), pp.905-917.
Divi, R.L. and Doerge, D.R., 1996. Inhibition of thyroid peroxidase by dietary flavonoids. Chemical research in toxicology, 9(1), pp.16-23.
Divi, R.L., Chang, H.C. and Doerge, D.R., 1997. Anti-thyroid isoflavones from soybean: isolation, characterization, and mechanisms of action. Biochemical pharmacology, 54(10), pp.1087-1096.
Dodd, M.H.I. and Dodd, J.M., 1976. The biology of metamorphosis. Physiology of the Amphibia, 3, pp.467-599.
Doerge, D.R. and Chang, H.C., 2002. Inactivation of thyroid peroxidase by soy isoflavones, in vitro and in vivo. Journal of Chromatography B, 777(1), pp.269-279.
Friedman, K.P., Watt, E.D., Hornung, M.W., Hedge, J.M., Judson, R.S., Crofton, K.M., Houck, K.A. and Simmons, S.O., 2016. Tiered High-Throughput Screening Approach to Identify Thyroperoxidase Inhibitors Within the ToxCast Phase I and II Chemical Libraries. Toxicological Sciences, p.kfw034.
Gereben, B., Zavacki, A.M., Ribich, S., Kim, B.W., Huang, S.A., Simonides, W.S., Zeold, A. and Bianco, A.C., 2008a. Cellular and molecular basis of deiodinase-regulated thyroid hormone signaling 1. Endocrine reviews, 29(7), pp.898-938.
Gereben, B., Zeöld, A., Dentice, M., Salvatore, D. and Bianco, A.C., 2008b. Activation and inactivation of thyroid hormone by deiodinases: local action with general consequences. Cellular and Molecular Life Sciences, 65(4), pp.570-590.
Haselman, J.T., Olker, J.H., Kosian, P.A., Korte, J.J., Swintek, J.A., Denny, J.S., Nichols, J.W., Tietge, J.E., Hornung, M.W. and Degitz, S.J., 2020. Targeted pathway-based in vivo testing using thyroperoxidase inhibition to evaluate plasma thyroxine as a surrogate metric of metamorphic success in model amphibian Xenopus laevis. Toxicological Sciences, 175(2), pp.236-250.
Hennemann, G., Docter, R., Friesema, E.C., de Jong, M., Krenning, E.P. and Visser, T.J., 2001. Plasma membrane transport of thyroid hormones and its role in thyroid hormone metabolism and bioavailability. Endocrine reviews, 22(4), pp.451-476.
Hornung, M.W., Kosian, P.A., Haselman, J.T., Korte, J.J., Challis, K., Macherla, C., Nevalainen, E. and Degitz, S.J., 2015. In vitro, ex vivo, and in vivo determination of thyroid hormone modulating activity of benzothiazoles. Toxicological Sciences, 146(2), pp.254-264.
Kessler, J., Obinger, C. and Eales, G., 2008. Factors influencing the study of peroxidase-generated iodine species and implications for thyroglobulin synthesis. Thyroid, 18(7), pp.769-774.
Marinovich, M., Guizzetti, M., Ghilardi, F., Viviani, B., Corsini, E. and Galli, C.L., 1997. Thyroid peroxidase as toxicity target for dithiocarbamates. Archives of toxicology, 71(8), pp.508-512.
OECD. (2009). Test No. 231: Amphibian Metamorphosis Assay, OECD Guidelines for the Testing of Chemicals, Section 2. OECD Publishing, Paris.
OECD. (2015). Test No. 241: The Larval Amphibian Growth and Development Assay (LAGDA), OECD Guidelines for the Testing of Chemicals, Section 2. OECD Publishing, Paris.
Paul, K.B., Hedge, J.M., Macherla, C., Filer, D.L., Burgess, E., Simmons, S.O., Crofton, K.M. and Hornung, M.W., 2013. Cross-species analysis of thyroperoxidase inhibition by xenobiotics demonstrates conservation of response between pig and rat. Toxicology, 312, pp.97-107.
Paul, K.B., Hedge, J.M., Rotroff, D.M., Hornung, M.W., Crofton, K.M. and Simmons, S.O., 2014. Development of a thyroperoxidase inhibition assay for high-throughput screening. Chemical research in toxicology, 27(3), pp.387-399.
Ruf, J. and Carayon, P., 2006. Structural and functional aspects of thyroid peroxidase. Archives of biochemistry and biophysics, 445(2), pp.269-277.
Schreiber, A.M. and Specker, J.L., 1998. Metamorphosis in the summer flounder (Paralichthys dentatus): stage-specific developmental response to altered thyroid status. General and comparative endocrinology, 111(2), pp.156-166.
Schmutzler, C., Bacinski, A., Gotthardt, I., Huhne, K., Ambrugger, P., Klammer, H., Schlecht, C., Hoang-Vu, C., Grüters, A., Wuttke, W. and Jarry, H., 2007. The ultraviolet filter benzophenone 2 interferes with the thyroid hormone axis in rats and is a potent in vitro inhibitor of human recombinant thyroid peroxidase. Endocrinology, 148(6), pp.2835-2844.
Shi, Y.B., Wong, J., Puzianowska‐Kuznicka, M. and Stolow, M.A., 1996. Tadpole competence and tissue‐specific temporal regulation of amphibian metamorphosis: Roles of thyroid hormone and its receptors. Bioessays, 18(5), pp.391-399.
Shi, Y.B., 2000. Amphibian metamorphosis. Wiley-Liss.
Taurog, A., Dorris, M.L. and Doerge, D.R., 1996. Mechanism of simultaneous iodination and coupling catalyzed by thyroid peroxidase. Archives of biochemistry and biophysics, 330(1), pp.24-32.
Tietge, J.E., Butterworth, B.C., Haselman, J.T., Holcombe, G.W., Hornung, M.W., Korte, J.J., Kosian, P.A., Wolfe, M. and Degitz, S.J., 2010. Early temporal effects of three thyroid hormone synthesis inhibitors in Xenopus laevis. Aquatic Toxicology, 98(1), pp.44-50.
Tietge, J.E., Degitz, S.J., Haselman, J.T., Butterworth, B.C., Korte, J.J., Kosian, P.A., Lindberg-Livingston, A.J., Burgess, E.M., Blackshear, P.E. and Hornung, M.W., 2013. Inhibition of the thyroid hormone pathway in Xenopus laevis by 2-mercaptobenzothiazole. Aquatic toxicology, 126, pp.128-136.
U.S. EPA. (2009). OCSPP 890.1100: Amphibian Metamorphosis Assay (AMA), Endocrine Disruptor Screening Program Test Guidelines, 890 Series. Available at: www.regulations.gov, ID: EPA-HQ-OPPT-2009-0576-0002. Accessed March 20, 2020.
U.S. EPA. (2015). OCSPP 890.2300: Larval Amphibian Growth and Development Assay (LAGDA), Endocrine Disruptor Screening Program Test Guidelines, 890 Series. Available at: www.regulations.gov, ID: EPA-HQ-OPPT-2014-0766-0020. Accessed March 20, 2020.
Visser, W.E., Friesema, E.C. and Visser, T.J., 2011. Minireview: thyroid hormone transporters: the knowns and the unknowns. Molecular endocrinology, 25(1), pp.1-14.
Wu, Y. and Koenig, R.J., 2000. Gene regulation by thyroid hormone. Trends in Endocrinology & Metabolism, 11(6), pp.207-211.
Yen, P.M., Ando, S., Feng, X., Liu, Y., Maruvada, P. and Xia, X., 2006. Thyroid hormone action at the cellular, genomic and target gene levels. Molecular and cellular endocrinology, 246(1), pp.121-127.
Zoeller, R.T., Tan, S.W. and Tyl, R.W., 2007. General background on the hypothalamic-pituitary-thyroid (HPT) axis. Critical reviews in toxicology, 37(1-2), pp.11-53.