
The authors have designated this AOP as all rights reserved. Re-use in any form requires advanced permission from the authors.
AOP: 374
Title
Binding of Sars-CoV-2 spike protein to ACE 2 receptors expressed on brain cells (neuronal and non-neuronal) leads to neuroinflammation resulting in encephalitis
Short name
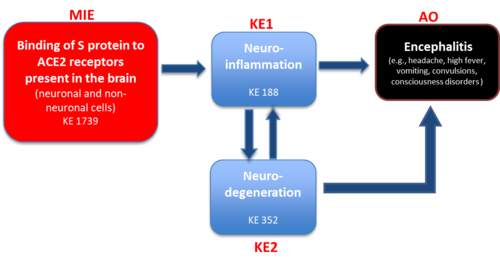
Graphical Representation
Point of Contact
Contributors
- Anna Price
- Francesca Pistollato
Coaches
- Cinzia La Rocca
OECD Information Table
| OECD Project # | OECD Status | Reviewer's Reports | Journal-format Article | OECD iLibrary Published Version |
|---|---|---|---|---|
| 1.96 | Under Development |
This AOP was last modified on April 29, 2023 16:03
Revision dates for related pages
| Page | Revision Date/Time |
|---|---|
| Binding to ACE2 | October 21, 2024 02:25 |
| Neuroinflammation | July 15, 2022 09:54 |
| N/A, Neurodegeneration | February 23, 2021 05:07 |
| Encephalitis | February 23, 2021 05:22 |
| Binding to ACE2 leads to Neuroinflammation | February 23, 2021 05:33 |
| Neuroinflammation leads to N/A, Neurodegeneration | February 23, 2021 05:47 |
| N/A, Neurodegeneration leads to Encephalitis | February 23, 2021 06:04 |
| Neuroinflammation leads to Encephalitis | February 23, 2021 06:17 |
| Sars-CoV-2 | September 09, 2022 05:09 |
| Virus | May 29, 2018 07:10 |
| bacteria | February 23, 2021 05:15 |
Abstract
Although the severe acute respiratory syndrome COVID-19 caused by the virus SARS-CoV-2 mainly manifests as an effect of acute respiratory infection (Radnis et al. 2020), recent evidence also suggests that approximately 36% of affected patients exhibit neurological sequelae (Mao et al., 2020).
Many studies have shown that SARS-CoV-2 coronavirus is neuroinvasive, neurotropic, and neurovirulent in humans. The presence of SARS-CoV-2 has been identified in the central nervous system (CNS) and cerebrospinal fluid (CSF) of patients with acute neurologic symptoms, including encephalitis (Wu et al., 2020). Post-mortem examination of SARS-CoV-2-infected patients revealed the presence of SARS-CoV-2 viral particles in endothelial cells and pericytes of brain capillaries and neurons (Paniz-Mondolfi et al., 2020; Nath, 2020; Chigr et al. 2020) as well as in glial cells (microglia and astrocytes) (Vargas et al. 2020; Nakagaki et al., 2005; Lannes et al., 2017).
There are several routes of Sars-CoV-2 entry into the CNS. The presence of viral-like particles of SARS-CoV-2 in endothelial cells and pericytes of brain capillaries, as well as astrocytic processes, strongly supports a hematogenous endothelial neuroinvasion-based hypothesis (Paniz-Mondolfi et al., 2020). Indeed, SARS-CoV-2 induced over-activation of systemic immune cells and the release of pro-inflammatory chemokines and cytokines, provokes a cytokine storm possibly leading to the to disruption of the tight junctions and increased permeability of the blood brain barrier (BBB) (Savarin and Bergmann 2018).
In parallel, SARS-CoV-2 could also infect the endothelial cells of the blood-cerebrospinal fluid barrier, and then spread into the CNS. Olfactory pathway (through nasal epithelium), vagus and trigeminal nerves, as well as the enteric nervous system are other possible routes of probable Sars-CoV-2 entry into the CNS.
Binding of S protein to ACE2 receptors on microglia triggers their activation, which results in the release of proinflamatory cytokines/chemokines, nitric oxide, prostaglandin E2, and reactive oxygen and nitrogen species. As a consequence, activation of astrocytes occurs, which ultimately may lead to neuronal cell death (Ransohoff and Perry, 2009; Chatterjee et al., 2013; Wheeler et al., 2018). This proinflamatory factors can trigger neuronal cell death by well-known mechanisms contributing, together with brain neuroinflammation, to encephalitis.
Encephalitis has been documented in clinical observations in COVID-19 patients, characterized by acute onset and common symptoms include headache, fever, vomiting, convulsions, and consciousness disorders (Wu Y et al., 2020). In the ongoing pneumonia epidemic, the presence of Sars-CoV-2 in the CSF of patients with COVID-19 was confirmed by genome sequencing, thereby clinically verifying viral encephalitis (Xiang et al., 2020), providing a solid basis for Sars-CoV-2 as a cause of this disease.
The present AOP describes current mechanistic understanding of causative links between binding of Spike protein to ACE2 receptors on brain cells, which leads to glia activation, neuronal inflammation and cell death, resulting in encephalitis (adverse outcome), which manifests through a variety of symptoms including headache, high fever, vomiting, convulsions, and consciousness disorders, as observed in several clinical studies in COVID-19 patients.
AOP Development Strategy
Context
Two KEs of this AOP (i.e., neuroinflammation and neurodegeneration) have been described in other AOPs (i.e., neuroinflammation (in AOP 12, AOP 48, AOP 3, and AOP 17); neurodegeneration (in AOP 12, AOP 48, AOP 281); however, quantitative information of KERs is limited or non-existing.
Strategy
Summary of the AOP
Events:
Molecular Initiating Events (MIE)
Key Events (KE)
Adverse Outcomes (AO)
| Type | Event ID | Title | Short name |
|---|
| MIE | 1739 | Binding to ACE2 | Binding to ACE2 |
| KE | 188 | Neuroinflammation | Neuroinflammation |
| KE | 352 | N/A, Neurodegeneration | N/A, Neurodegeneration |
| AO | 1841 | Encephalitis | Encephalitis |
Relationships Between Two Key Events (Including MIEs and AOs)
| Title | Adjacency | Evidence | Quantitative Understanding |
|---|
| Binding to ACE2 leads to Neuroinflammation | adjacent | High | Low |
| Neuroinflammation leads to N/A, Neurodegeneration | adjacent | High | Not Specified |
| N/A, Neurodegeneration leads to Encephalitis | adjacent | Low | Low |
| Neuroinflammation leads to Encephalitis | adjacent | High | Low |
Network View
Prototypical Stressors
| Name |
|---|
| Sars-CoV-2 |
| Virus |
| bacteria |
Life Stage Applicability
| Life stage | Evidence |
|---|---|
| Adults | High |
Taxonomic Applicability
| Term | Scientific Term | Evidence | Link |
|---|---|---|---|
| human | Homo sapiens | High | NCBI |
Sex Applicability
| Sex | Evidence |
|---|---|
| Mixed | High |
Overall Assessment of the AOP
Domain of Applicability
Essentiality of the Key Events
Essentiality for MIE:
SARS-CoV-2 was able to infect and kill neural cells, including cortical neurons. This phenotype was accompanied by impaired synaptogenesis. Sofosbuvir, an FDA-approved antiviral drug, was able to rescue these alterations (Mesci et al, 2020). It is speculated that the viral mediated production of autoantibodies against glial cells might be responsible for neural injury (Zanin et al., 2020). Neural infection can be prevented by using either anti-ACE2 antibodies or anti-spike antibodies from the cerebrospinal fluid (CSF) of COVID-19 patients (Mesci et al., 2020). Organoids incubated with either anti-ACE2 antibodies or anti-spike antibodies from the CSF of COVID-19 patients saw a significant decrease in SARS-CoV-2 infection. Likewise, transgenic mice overexpressing human ACE2 were used to demonstrate viral replication in the brain and the lethal consequences of SARS-CoV-2 CNS infection. SARS-CoV-2 infected mice overexpressing human ACE2 showed increase viral titers in the brain and death associated to neuroinvasion (Song et al., 2020).
Evidence Assessment
Known Modulating Factors
Quantitative Understanding
Considerations for Potential Applications of the AOP (optional)
References
AOP 12 Chronic binding of antagonist to N-methyl-D-aspartate receptors (NMDARs) during brain development leads to neurodegeneration with impairment in learning and memory in aging. Available at https://aopwiki.org/aops/12
AOP 17 Binding of electrophilic chemicals to SH(thiol)-group of proteins and /or to seleno-proteins involved in protection against oxidative stress during brain development leads to impairment of learning and memory. Available at https://aopwiki.org/aops/17
AOP 281 Acetylcholinesterase Inhibition Leading to Neurodegeneration. Available at https://aopwiki.org/aops/281
AOP 3 Inhibition of the mitochondrial complex I of nigro-striatal neurons leads to parkinsonian motor deficits. Available at https://aopwiki.org/aops/3
AOP 48 Binding of agonists to ionotropic glutamate receptors in adult brain causes excitotoxicity that mediates neuronal cell death, contributing to learning and memory impairment. Available at https://aopwiki.org/aops/48
Chatterjee D, et al. Microglia play a major role in direct viral-induced demyelination. Clin Dev Immunol. 2013; 2013():510396.
Chigr F., et al. Comment on “The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients”. J Med Virol. 2020 Jul;92(7):703-704.
Lannes N., Neuhaus V., Scolari B., Kharoubi-Hess S., Walch M., Summerfield A., Filgueira L. Interactions of human microglia cells with Japanese encephalitis virus. Virol. J. 2017;14(1):8.
Mao et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurology (2020) 77(6): 683–690.
Mesci P et al. Sofosbuvir protects human brain organoids against SARS-CoV-2. bioRxiv. 2020. Available at: doi: https://doi.org/10.1101/2020.05.30.125856
Nakagaki K., Nakagaki K., Taguchi F. Receptor-independent spread of a highly neurotropic murine coronavirus JHMV strain from initially infected microglial cells in mixed neural cultures. J. Virol. 2005;79(10):6102–6110.
Nath A, Smith B. Neurological issues during COVID-19: An overview. Neurosci Lett. 2021 Jan 18;742:135533.
Paniz-Mondolfi et al. Central nervous system involvement by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). Med Virol. 2020 Jul;92(7):699-702.
Radnis C, et al. Radiographic and clinical neurologic manifestations of COVID-19 related hypoxemia. J Neurol Sci. 2020 Nov 15;418:117119. Gallagher et al., 2006;
Ransohoff RM, Perry VH. Microglial physiology: unique stimuli, specialized responses. Annu Rev Immunol. 2009; 27():119-45.
Savarin C, Bergmann CC. Fine Tuning the Cytokine Storm by IFN and IL-10 Following Neurotropic Coronavirus Encephalomyelitis. Front Immunol. 2018 Dec 20;9:3022.
Song et al. Neuroinvasive potential of SARS-CoV-2 revealed in a human brain organoid model. bioRxiv. 2020. Available at: https://www.biorxiv.org/content/10.1101/2020.06.25.169946v1
Vargas G. et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and glial cells: Insights and perspectives. Brain Behav Immun Health. 2020 Aug; 7: 100127.
Wheeler DL, et al. Microglia are required for protection against lethal coronavirus encephalitis in mice. J Clin Invest. 2018 Mar 1; 128(3):931-943.
Wu Y, et al. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav Immun. 2020 Jul;87:18-22.
Xiang YT, et al. The COVID-19 outbreak and psychiatric hospitals in China: managing challenges through mental health service reform. Int J Biol Sci. 2020 Mar 15;16(10):1741-1744.
Zanin L, et al. SARS-CoV-2 can induce brain and spine demyelinating lesions. Acta Neurochir (Wien). 2020 Jul;162(7):1491-1494.