
This AOP is licensed under the BY-SA license. This license allows reusers to distribute, remix, adapt, and build upon the material in any medium or format, so long as attribution is given to the creator. The license allows for commercial use. If you remix, adapt, or build upon the material, you must license the modified material under identical terms.
AOP: 42
Title
Inhibition of Thyroperoxidase and Subsequent Adverse Neurodevelopmental Outcomes in Mammals
Short name
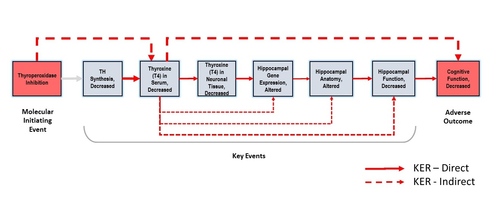
Graphical Representation
Point of Contact
Contributors
- Kevin Crofton
- Mary Gilbert
- Katie Paul Friedman
- Anna Price
Coaches
OECD Information Table
| OECD Project # | OECD Status | Reviewer's Reports | Journal-format Article | OECD iLibrary Published Version |
|---|---|---|---|---|
| 1.10 | WPHA/WNT Endorsed | Scientific Review | iLibrary link |
This AOP was last modified on April 29, 2023 16:02
Revision dates for related pages
| Page | Revision Date/Time |
|---|---|
| Thyroperoxidase, Inhibition | November 18, 2025 08:29 |
| Thyroid hormone synthesis, Decreased | November 04, 2022 09:25 |
| Thyroxine (T4) in serum, Decreased | October 10, 2022 08:52 |
| Thyroxine (T4) in neuronal tissue, Decreased | April 04, 2019 09:13 |
| Cognitive function, decreased | July 25, 2024 17:23 |
| Hippocampal gene expression, Altered | August 11, 2018 09:26 |
| Hippocampal anatomy, Altered | July 24, 2024 23:09 |
| Hippocampal Physiology, Altered | July 24, 2024 23:15 |
| Thyroperoxidase, Inhibition leads to TH synthesis, Decreased | November 04, 2022 09:27 |
| TH synthesis, Decreased leads to T4 in serum, Decreased | October 10, 2022 08:56 |
| Thyroperoxidase, Inhibition leads to T4 in serum, Decreased | November 18, 2025 08:50 |
| T4 in serum, Decreased leads to T4 in neuronal tissue, Decreased | April 04, 2019 10:50 |
| T4 in neuronal tissue, Decreased leads to Hippocampal gene expression, Altered | August 11, 2018 19:18 |
| Hippocampal gene expression, Altered leads to Hippocampal anatomy, Altered | August 11, 2018 19:05 |
| Hippocampal anatomy, Altered leads to Hippocampal Physiology, Altered | July 25, 2024 18:38 |
| Hippocampal Physiology, Altered leads to Cognitive function, decreased | July 26, 2024 12:57 |
| T4 in serum, Decreased leads to Hippocampal gene expression, Altered | August 11, 2018 19:40 |
| T4 in serum, Decreased leads to Hippocampal anatomy, Altered | August 11, 2018 19:34 |
| T4 in serum, Decreased leads to Hippocampal Physiology, Altered | August 11, 2018 19:44 |
| T4 in serum, Decreased leads to Cognitive function, decreased | August 11, 2018 19:44 |
| Methimazole | November 29, 2016 18:42 |
| Propylthiouracil | November 29, 2016 18:42 |
Abstract
This AOP describes one adverse outcome that may result from the inhibition of thyroperoxidase (TPO) during mammalian development. Chemical inhibition of TPO, the molecular-initiating event (MIE), results in decreased thyroid hormone (TH) synthesis, and subsequent reduction in circulating concentrations of THs. THs are essential for normal human brain development, both prenatally and postnatally, modulating genes critical for a normal neuroanatomical development, with subsequent effects on neurophysiology, and finally neurological function. Therefore, chemicals that interfere with TH synthesis have the potential to cause TH insufficiency that may result in adverse neurodevelopmental effects in offspring. Herein, we discuss the implications of developmental TPO inhibition for hippocampal anatomy, function, and ultimately neural function controlled by the hippocampus. The biochemistry of TPO and its essentiality for TH synthesis is well known across species. The hippocampus is known to be critically involved in cognitive, emotional, and memory function. The adverse consequences of TH insufficiency depend both on severity and developmental timing, indicating that exposure to TPO inhibitors may produce different effects at different developmental windows of exposure. It is important to note that thyroid stimulating hormone (TSH) is not a KE in this AOP. While TSH may play a role in feedback-driven compensatory processes, it is not directly involved in brain development. The overall weight of evidence for this AOP is strong. Gaps in our understanding include the relationship of TH-dependent gene expression and complexities of brain development. Although quantitative information at all levels of KERs is limited a number of applications of this AOP have been identified.
AOP Development Strategy
Context
This AOP was originally started on the Chemical Mode of Action WIKI sponsored by WHO/IPCS. The MOA was originally described and published by Zoeller and Crofton (Crit Rev Toxicol 2005). Thanks to the following contributors whose work on the MOA-WIKI fostered further development on the AOP wiki: Michelle Embry, Richard Judson, Vicki Dellarco, Chihae Yang, Kevin Crofton.
Zoeller RT, Crofton KM. Mode of action: developmental thyroid hormone insufficiency--neurological abnormalities resulting from exposure to propylthiouracil. Crit Rev Toxicol. 2005 Oct-Nov;35(8-9):771-81
Strategy
Summary of the AOP
Events:
Molecular Initiating Events (MIE)
Key Events (KE)
Adverse Outcomes (AO)
| Type | Event ID | Title | Short name |
|---|
| MIE | 279 | Thyroperoxidase, Inhibition | Thyroperoxidase, Inhibition |
| KE | 277 | Thyroid hormone synthesis, Decreased | TH synthesis, Decreased |
| KE | 281 | Thyroxine (T4) in serum, Decreased | T4 in serum, Decreased |
| KE | 280 | Thyroxine (T4) in neuronal tissue, Decreased | T4 in neuronal tissue, Decreased |
| KE | 756 | Hippocampal gene expression, Altered | Hippocampal gene expression, Altered |
| KE | 757 | Hippocampal anatomy, Altered | Hippocampal anatomy, Altered |
| KE | 758 | Hippocampal Physiology, Altered | Hippocampal Physiology, Altered |
| AO | 402 | Cognitive function, decreased | Cognitive function, decreased |
Relationships Between Two Key Events (Including MIEs and AOs)
| Title | Adjacency | Evidence | Quantitative Understanding |
|---|
| Thyroperoxidase, Inhibition leads to TH synthesis, Decreased | adjacent | High | Low |
| TH synthesis, Decreased leads to T4 in serum, Decreased | adjacent | High | Moderate |
| T4 in serum, Decreased leads to T4 in neuronal tissue, Decreased | adjacent | Moderate | Moderate |
| T4 in neuronal tissue, Decreased leads to Hippocampal gene expression, Altered | adjacent | Moderate | Low |
| Hippocampal gene expression, Altered leads to Hippocampal anatomy, Altered | adjacent | Moderate | Low |
| Hippocampal anatomy, Altered leads to Hippocampal Physiology, Altered | adjacent | Moderate | Low |
| Hippocampal Physiology, Altered leads to Cognitive function, decreased | adjacent | High | Moderate |
| Thyroperoxidase, Inhibition leads to T4 in serum, Decreased | non-adjacent | High | Moderate |
| T4 in serum, Decreased leads to Hippocampal gene expression, Altered | non-adjacent | High | Low |
| T4 in serum, Decreased leads to Hippocampal anatomy, Altered | non-adjacent | High | Low |
| T4 in serum, Decreased leads to Hippocampal Physiology, Altered | non-adjacent | Moderate | Low |
| T4 in serum, Decreased leads to Cognitive function, decreased | non-adjacent | High | Moderate |
Network View
Prototypical Stressors
Life Stage Applicability
| Life stage | Evidence |
|---|---|
| During brain development | High |
| Development | High |
Taxonomic Applicability
Sex Applicability
| Sex | Evidence |
|---|---|
| Male | High |
| Female | High |
Overall Assessment of the AOP

The following summary tables for:
1.Support for Biological Plausibility of KERS
2. Support for Essentiality of KEs
3. Empirical Support for KERs
Can be downloaded at: https://aopwiki.org/system/dragonfly/production/2018/08/10/46w2o2kkl4_TPO_AOP_Summary_Tables_20180602.pdf
Domain of Applicability
- Chemicals: This AOP applies to a wide range of chemicals structures that inhibit TPO either in vivo or in vitro. Well recognized positive controls include propylthiouracil (PTU) and methimazole (MMI). There are 100s of other chemicals known to inhibit TPO in vitro (e.g., Paul-Friedman et al., 2016).
- Sex: This AOP applies to males and females. Disruption of thyroid hormone regulation during fetal and early postnatal develop, as well as the subsequent adverse impacts on nervous system development are similar in both sexes. There are no compelling data to suggest sex differences in susceptibility to TH disruption mediated by inhibition of TPO during development.
- Life stages: The relevant life stages for this AOP are fetal and early postnatal ages during critical windows of nervous system development where thyroid hormones guide normal development of the brain. There are clear windows of developmental susceptibility and different brain regions show distinct ontogenetic profiles for TH requirements. Distinct phenotypes have been described in both humans and animal models for different periods of TH insufficiency. The influence of maternal thyroid status prior to onset of fetal thyroid function is an important consideration. This AOP does not apply to adult life states.
- Taxonomic: Based on the majority of the available evidence the taxonomic applicability domains of this AOP is mammals. Most evidence for this AOP has been gathered primarily from laboratory rodents and humans. However, there are supporting data from amphibians and birds for TPO inhibition leading to altered TH profiles. Due to the conserved nature of TH synthesis, transport, metabolism and transcriptional activity, this AOP is likely to be applicable to other classes of vertebrates where thyroid hormones drive development of the nervous system (e.g., birds, fish, reptiles). However, species-specific differences in development, ADME (adsorption, distribution, metabolism, and elimination), compensatory endocrine responses may influence the outcomes, particularly from a quantitative standpoint.
Essentiality of the Key Events
It is widely accepted that each of the key events is essential.
- Molecular Initiating Event: The molecular initiating event, i.e. inhibition of TPO, is the essential event to initiate this AOP, as supported by in vitro and in vivo evidence. TPO is the only enzyme capable of de novo TH synthesis (Taurog, 2005). TPO is classically defined as a complex enzyme with multiple catalytic cycles capable of iodinating multiple species (Divi et al., 1997). However, in the context of this AOP we are using TPO inhibiton not in the classical sense, but instead to refer to the results derived from the assays commonly used assays to investigate environmental chemicals (e.g., guiacol oxidation). A number of studies have demonstrated that cessation of exposure is known to result in a return to normal levels of TH synthesis and circulatory hormone levels (Cooper et al., 1983). Many in vivo and in vitro studies consistently demonstrate enzyme inhibition with similar chemicals for multiple species (Taurog, 1999; Paul et al., 2013; Vickers et al., 2012).
- Thyroid hormone synthesis, Decreased. A number of studies have demonstrated a correlation between TPO activity and decreased TH synthesis (e.g., Vickers et al., 2012). Thyroid gland T4 concentrations, as well as serum TH, are decreased in response to thyroidectomy and recover when in-vitro derived follicles are grafted in athyroid mice (Antonica et al., 2012).
- Thyroxine (T4) in serum, Decreased. Inhibition of TPO is widely accepted as resulting in decreased TH synthesis in the thyroid gland, which results in decreased serum T4 concentration (Taurog, 2005). Stop/recovery experiments demonstrate recovery of serum thyroxine concentrations due to cessation of developmental exposure to chemical stressors (e.g., Crofton et al., 2000), with similar findings in adult rats (Cooper et al.,1984). Studies in adult animals show a similar recovery after cessation of dosing (e.g., Hill et al., 1998).
- Thyroxine (T4) in neuronal tissue, decreased: Mulitple studies have demonstrated that fetal brain TH levels, previously decreased by maternal exposure to TPO inhibitors or thyroidectomy, recovered following maternal dosing with T4 (e.g., Calvo et al., 1990). In addition, upregulation of deiodinase has been shown compensate for some loss of neuronal T3 (Escobar-Morreale et al., 1997). Indirect evidence shows that T4 replacement that bring circulating T4 concentration back to normal, leads to recovery of brain TH and prevents downstream effects including alterations in gene expression in the developing brain.
- Hippocampal Gene Expression, Altered: It is well established specific genomic pathways underlie the progression of a number of neurodevelopmental processes in the hippocampus. There is some evidence from ex vivo studies that administration of growth factors will reverse the hippocampal dysplasia seen in Jacob/Nsfm knockout mice (Spilker et al., 2016). Less is known about the impact of hormone replacement on TH-responsive gene expression and the qualitative and quantitative relationships between altered TH-dependent gene expression in this brain region and altered hippocampal cytoarchitectural anatomy.
- Hippocampal anatomy, altered: It is well accepted that normal hippocampal anatomy is critical for hippocampal physiological function, and that alterations in anatomy lead to altered neuronal activity in the hippocampus (Lee et al., 2015; Grant et al., 1992; Spilker et al., 2016).
- Hippocampal physiology, altered: It is a well-accepted assertion that hippocampal synaptic integrity and neuronal plasticity are essential for spatial information processing in animals and spatial and episodic memory in humans. However, other brain regions also can influence these complex behaviors. Limited data from studies in BDNF knockout animals demonstrate that deficits in hippocampal synaptic transmission and plasticity, and downstream behaviors can be rescued with recombinant BDNF (Aarse et al., 2016; Andero et al., 2014).
- Cognitive function, decreased: It is a well-known fact that TH are critical for normal nervous system development (Williams et al., 2008). And this includes development of the hippocampus which plays a major role in spatial, temporal, and contextual memory. Indeed, most developed countries check for childhood hypothyroidism at birth to immediately begin replacement therapy. This has been shown to alleviate most adverse impacts of hypothyroidism in congenitally hypothyroid children (Derksen-Lubsen and Verkerk 1996; Zoeller and Rovet, 2004). The essentiality of the relationship between decreased TH levels and this adverse outcome is well accepted. Decreased cognitive function specific to the hippocampal region are particularly associated with decrements in memory and learning domains of cognition.
Evidence Assessment
Biological plausibility: Biological plausibility refers to the structural or functional relationship between the key events based on our fundamental understanding of "normal biology". In general, the biological plausibility and coherence linking TPO inhibition through decreases in circulating concentrations of THs, to adverse impacts in the developing hippocampus and subsequent cognitive behaviors is very solid. That thyroidal TPO is the sole enzyme capable of de novo TH synthesis and the only source of circulating T4, is beyond doubt. It is also widely accepted that circulating T4 is the only source of nervous system T4 that is converted to the biological active T3. The direct link between reduced brain TH concentrations and reduced expression of TR regulated genes is supported by a plethora of literature. However, the direct connection between exactly which genes are regulated and at which developmental periods is not as clear. Similarly, the precise relationships between gene expression and hippocampal anatomy is not completely known. A lot of the work in this area has been done for a limited number of genes and specific hippocampal anatomical anomalies that are known to alter both the physiological and function of the hippocampus, and subsequent cognitive function. That said, it is widely acknowledged that abnormal TH levels during fetal and early development lead to adverse hippocampally-driven cognitive function in humans and laboratory animals.
- The biochemistry of TPO and its essentiality for TH synthesis is well known across species, with the evidence across vertebrate species, including amphibians, birds, rodents, pigs, and humans.
- The relationship between TH synthesis and serum TH concentrations is well accepted scientific dogma. There are no other pathways in mammals that will maintain homeostatic serum TH concentrations.
- Serum is the only source of thyroxine for the brain. In the brain, deiodinases convert T4 to T3, the more biologically active moiety. Some serum T3 may also contribute to total brain T3. These are well accepted scientific facts.
- It is well established that T3 binding to thyroid receptors controls critical transcriptional and translational processes in the developing brain, including the hippocampus. Lack of TH results in abnormal development of the structure and physiological function in the hippocampus. What is not well known is exactly which genes, at what fetal and postnatal ages, are responsible for the development of the complexity of hippocampal anatomy and function.
- Lastly, the biological plausibility that changes in brain structure and physiology, and specifically aberrations in the hippocampus, lead to abnormal cognitive function is well accepted.
Concordance of dose-response relationships:
There are a large number of studies that include correlative evidence between exposure to TPO inhibitors and downstream KEs, as well as the AO. In addition, there are also studies with dose-response relationships that indirectly link KEs, especially from serum TH concentrations to downstream KEs and the AO. There is a more limited set of studies in which two directly linked key events were considered in the same study following exposure to TPO inhibitors or other stressors (e.g., thyroidectomy, gene knockouts). These later studies, while providing critical data for causatively linking the key events, provide less information on the concordance of the dose-response relationship, especially for the latter KEs. For earlier KEs, Zoeller and Crofton (2005) provide good dose response concordance for data derived from the TPO inhibitor PTU. While limited in number, in general these studies provide moderate confidence that downstream key events occurred at concentrations equal to or greater than those directly upstream. In addition, there are several quantitiative models that, based on empirical data, can predict dose relationships between many of the early KEs up to and including serum hormone concentrations (e.g., Degon et al., 2008; Fisher et al 2013; Ekerot et al., 2012; Leonard et al., 2016). A more recent model predicts neuroanatomical anomalies based on serum and brain T4 concentrations (Hassan et al., 2017).
All this information taken together, provide strong concordance of the dose-relationships for all KEs.
Temporal concordance among the key events and adverse effect: There are two aspects of the temporal concordance of the key events in a developmental AOP. The first is the temporal concordance refers to the degree to which the data support the hypothesized sequence of the key events; i.e., the effect on KE1 is observed before the effect on KE2, which is observed before the effect on KE3, and so on. This translates to the temporal concordance of the AOP from TPO inhibition to decreased TH synthesis, reduced circulating TH concentrations, decreased nervous system TH, altered gene expression and anatomy in the hippocampus, and subsequent alterations in hippocampal physiology that result in decrements in cognition. The strength of the temporal concordance between these KEs varies from weak to strong (see Appendix Tables and individual KEs for detailed information). There is strong evidence for the early direct KEs from both empirical and modeling studies, and for many of the later KEs via the indirect KERs. The temporal concordance between TPO inhibition and TH synthesis is clearly evidenced by data from ex vivo and in vitro studies, as well as computational models (Leonard et al., 2016; Degon et al., 2008; Zoeller and Crofton, 2005; Cooper et al., 1983; Goldey et al. 1985; Christenson et al 1995). Data supporting the temporal concordance for the later KEs, i.e., from serum TH to changes in hippocampal physiology are limited or lacking.
The second aspect of temporal concordance for developmental AOPs is evidenced by demonstrations for critical windows of development where key events are perturbed, for which the effects are permanent and found during early development and throughout adulthood (Seed et al., 2005). It is a well-recognized fact that there are critical developmental windows for disruption of serum THs that result in subsequent alterations in all downstream KEs including the AO cognitive function later in development and adulthood. Indeed, the literature is replete with studies that demonstrate critical windows of susceptibity to thyroid disruption and adverse impacts on the developing brain. For reviews see: Morreale de Escobar (2001); Howdshell (2002). There are also many studies in which downstream direct and indirect consequences of TPO inhibition and other stressors (e.g., iodine deficiency, thyroidectomy, gene knockouts) have been ameliorated by administration of thyroxine. For example, based on the indirect link between serum TH hormone concentrations and decrements in hippocampally-mediated spatial behaviors, it commonly accepted dogma that there are critical windows of development in which exposure and hormone reduction lead to permanent effect on cognitive functions. Indeed, most developed countries have mandatory screening for congenital hypothyroidism, so that hormone replacement therapy can begin immediately, and thus prevent declines in IQ in childhood. (e.g., the temporal concordance between the MIE, KEs and AO. Overall, all available data are consistent with the temporal concordance of this AOP.
Consistency: There is no data that we are aware of that does not support the pattern of key events described in this AOP. A limited number of studies with measurements of directly linked KEs within the same study, the fact that the majority of the data was generated with single-stressor studies (e.g., one chemical dose, knockout, or thyroidectomy), coupled with likely differences in sensitivity of many of the measured endpoints (e.g., gene expression), make it difficult to determine quantitative consistency between studies. Nonetheless, the occurrence of the final AO, when upstream key events are observed is extremely consistent. It is also very important to note that the AO, alterations in cognitive function, is not likely to be specific solely to this AOP. Many of the key events included in this AOP overlap with AOPs linking other molecular initiating events to alterations in hippocampally-driven cognitive behaviors such as spatial learning in rats and IQ in humans.
Uncertainties, inconsistencies, and data gaps:
There are several areas of uncertainty and data gaps in the current AOP:
- There is a lack of quantitative information for several the KERs. These gaps hamper development of quantitative models that will allow linkages between the MIE and AO. Quantitative models are needed to facilitate efficient use of data on ~1000 chemicals from in vitro TPO assays (e.g., Paul-Friedman et al., 2016) to predict potential adverse outcomes. Computational models are needed to describe relationships between serum and brain TH as a critical KER. With an additional metric of TH action in brain, this may be sufficient for application to computational prediction in the regulatory arena. These gaps include:
- Insufficient information exists to quantitatively link the degree of in vivo TPO inhibition required to elicit specific decrements in circulating T4 concentrations; Genistein is an example of where a very large degree of inhibition may be required to have an impact on serum TH;
- There is a lack of data to quantitatively associate serum TH concentrations with TH concentrations in specific brain regions;
- Presently TH-responsive gene expression in hippocampus has not been quantitatively linked to changes in hippocampal anatomy, hippocampal function, and subsequent adverse cognitive effects. Neither has this AOP considered the nongenomic actions of TH on cell signaling in brain.
- There is limited available data that inform a quantitative relationship between in vitro and in vivo inhibition of TPO (but see Vickers et al., 2012).
- Compensatory feedback systems are not included in this AOP. For example, it is well known that with chemicals that inhibit TPO (e.g., PTU) decrease circulating TH concentrations which activates the hypothalamic-pituitary feedback system (Capen, 1997). This leads to increased secretion of TSH, which upregulates TH synthesis in the thyroid gland (e.g., McCain, 1995; Capen, 1997; Hill et al., 1998). There is also compensation within the developing nervous system where low tissue T4 concentrations upregulates deiodinases in an attempt to maintain proper levels of T3 (e.g., Morse et al., 1996; Sharlin et al 2010). These and other compensatory systems are likely to be differentially active across different developmental ages and in different brain regions
- Lastly, there is some uncertainty in the literature about the role of thyroid stimulating hormone (TSH) in thyroid hormone based adverse outcome pathways and the relevance of rodent data for humans. It is clear that TSH is a key event in the AOP for rat thyroid follicular tumors (McCain, 1995; Hill et al., 1998) and this pathway is not deemed relevant to humans (Axelrad et al., 2005). However, it is critically important to note that the current AOP does not contain TSH as a KE. This is because, while TSH may play a role in feedback-driven compensatory processes to maintain peripheral hormone concentrations, it is not directly involved in brain development. In this AOP, TSH may be used as a supporting biomarker for alterations in circulating THs, however, it is not a perfect surrogate. There are also numerous examples of pharmaceutical and industrial chemicals that alter circulating THs in rats without any measurable change in TSH (NTP, 1990; O’Connor et al., 1998 2000; Liu et al., 1994; Zoeller et al., 2005; Morse et al., 1996; Goldey et al., 1995; Lau et al 2003; Schneider et al., 2011). In the absence of TSH changes, exposure to some of these chemicals do result in adverse neurological outcomes (e.g., Goldey and Crofton, 1998; Crofton, 2004; Zoeller et al., 2005; Cope et al., 2015). Therefore, stressor-induced changes in TH, not in TSH, are responsible for adverse neurological outcomes.
Known Modulating Factors
Quantitative Understanding
Assessment of quantitative understanding of the AOP: Currently, there are quantitative models for the early KERs from TPO inhibiton to serum hormone concentrations, but none for later KERs. And only one of these models the KERs during early development (Fisher et al., 2013). A recent study by Hassan et al. (2017) quantitatively linked PTU-induced TH synthesis declines in the dam and the fetus to decrements in serum and brain TH concentrations to a structural malformation in the postnatal brain. In this study, estimates of TPO inhibition were derived from glandular and serum PTU and TH concentrations. For the rest of the KERs in this AOP, there is a varying amount of data from dose-response studies that demonstrate increasing impact with increasing chemical dose for all the KEs, and the direct and indirect KERs. At present, the overall quantitative understanding of the AOP is insufficient to directly link a measure of chemical potency as a TPO inhibitor to a quantitative prediction of effect on cognitive function (e.g., IQ in humans, learning deficits in rodents). Empirical information on dose-response relationships for the intermediate KEs, currently unavailable, would inform a computational, predictive model for thyroid disruption via TPO inhibition.
Considerations for Potential Applications of the AOP (optional)
References
Aarse J, Herlitze S, Manahan-Vaughan D. The requirement of BDNF for hippocampal synaptic plasticity is experience-dependent. Hippocampus. 2016 Jun;26(6):739-51.
Andero R, Choi DC, Ressler KJ. BDNF-TrkB receptor regulation of distributed adult neural plasticity, memory formation, and psychiatric disorders. Prog Mol Biol Transl Sci. 2014;122:169-92.
Antonica F, Kasprzyk DF, Opitz R, Iacovino M, Liao XH, Dumitrescu AM, Refetoff S, Peremans K, Manto M, Kyba M, Costagliola S. Generation of functional thyroid from embryonic stem cells. Nature. 2012 491(7422):66-71.
Axelrad DA, Baetcke K, Dockins C, Griffiths CW, Hill RN, Murphy PA, Owens N, Simon NB, Teuschler LK. Risk assessment for benefits analysis: framework for analysis of a thyroid-disrupting chemical. J Toxicol Environ Health A. 2005 68(11-12):837-55.
Calvo R, Obregón MJ, Ruiz de Oña C, Escobar del Rey F, Morreale de Escobar G. Congenital hypothyroidism, as studied in rats. Crucial role of maternal thyroxine but not of 3,5,3'-triiodothyronine in the protection of the fetal brain. J Clin Invest. 1990 Sep;86(3):889-99.
Capen CC Mechanistic data and risk assessment of selected toxic end points of the thyroid gland. Toxicol Pathol. 1997 Jan-Feb;25(1):39-48. Review.
Cooper, D.S., Kieffer, J.D., Halpern, R., Saxe, V., Mover, H., Maloof, F., and Ridgway, E.C. (1983). Propylthiouracil (PTU) pharmacology in the rat. II. Effects of PTU on thyroid function. Endocrinology 113:921–928.
Cooper DS, Kieffer JD, Saxe V, Mover H, Maloof F, Ridgway EC. Methimazole pharmacology in the rat: studies using a newly developed radioimmunoassay for methimazole. Endocrinology. 1984 Mar;114(3):786-93.
Cope RB, Kacew S, Dourson M. A reproductive, developmental and neurobehavioral study following oral exposure of tetrabromobisphenol A on Sprague-Dawley rats. Toxicology. 2015 329:49-59.
Crofton KM, Kodavanti PR, Derr-Yellin EC, Casey AC, Kehn LS. PCBs, thyroid hormones, and ototoxicity in rats: cross-fostering experiments demonstrate the impact of postnatal lactation exposure. Toxicol Sci. 2000 57(1):131-40.
Crofton KM. Developmental disruption of thyroid hormone: correlations with hearing dysfunction in rats. Risk Anal. 2004 Dec;24(6):1665-71.
Degon, M., Chipkin, S.R., Hollot, C.V., Zoeller, R.T., and Chait, Y. (2008). A computational model of the human thyroid. Mathematical Biosciences 212, 22–53
Derksen-Lubsen, G. and P. H. Verkerk (1996). "Neuropsychologic development in early treated congenital hypothyroidism: analysis of literature data." Pediatr Res 39(3): 561-6.
Ekerot P, Ferguson D, Glämsta EL, Nilsson LB, Andersson H, Rosqvist S, Visser SA. Systems pharmacology modeling of drug-induced modulation of thyroid hormones in dogs and translation to human. Pharm Res. 2013 30(6):1513-24.
Escobar-Morreale HF, Obregón MJ, Hernandez A, Escobar del Rey F, Morreale de Escobar G. Regulation of iodothyronine deiodinase activity as studied in thyroidectomized rats infused with thyroxine or triiodothyronine. Endocrinology. 1997 Jun;138(6):2559-68.
Fisher JW, Li S, Crofton K, Zoeller RT, McLanahan ED, Lumen A, Gilbert ME. Evaluation of iodide deficiency in the lactating rat and pup using a biologically based dose-response model. Toxicol Sci. 2013 132(1):75-86.
Goldey ES, Crofton KM. Thyroxine replacement attenuates hypothyroxinemia, hearing loss, and motor deficits following developmental exposure to Aroclor 1254 in rats. Toxicol Sci. 1998 45(1):94-10
Goldey ES, Kehn LS, Lau C, Rehnberg GL, Crofton KM. Developmental exposure to polychlorinated biphenyls (Aroclor 1254) reduces circulating thyroid hormone concentrations and causes hearing deficits in rats. Toxicol Appl Pharmacol. 1995; 135(1):77-88.
Grant SG, O'Dell TJ, Karl KA, Stein PL, Soriano P, Kandel ER. Impaired long-term potentiation, spatial learning, and hippocampal development in fyn mutant mice. Science. 1992 Dec 18;258(5090):1903-10.
Hassan, I, El-Masri, H., Kosian, PA, Ford, J, Degitz, SJ and Gilbert, ME. Quantitative Adverse Outcome Pathway for Neurodevelopmental Effects of Thyroid Peroxidase-Induced Thyroid Hormone Synthesis Inhibition. Toxicol Sci. 2017 Nov 1;160(1):57-73.
Howdeshell, K.L. A Model of the Development of the Brain as a Construct of the Thyroid System. Env Hlth Perpect. 2002. 100(suppl 3)337-348.
Hill RN, Crisp TM, Hurley PM, Rosenthal SL, Singh DV. Risk assessment of thyroid follicular cell tumors. Environ Health Perspect. 1998 Aug;106(8):447-57.
Lau C, Thibodeaux JR, Hanson RG, Rogers JM, Grey BE, Stanton ME, Butenhoff JL, Stevenson LA. Exposure to perfluorooctane sulfonate during pregnancy in rat and mouse. II: postnatal evaluation. Toxicol Sci. 2003 Aug;74(2):382-92
Lee KH, Lee H, Yang CH, Ko JS, Park CH, Woo RS, Kim JY, Sun W, Kim JH, Ho WK, Lee SH. Bidirectional Signaling of Neuregulin-2 Mediates Formation of GABAergicSynapses and Maturation of Glutamatergic Synapses in Newborn Granule Cells ofPostnatal Hippocampus. J Neurosci. 2015 Dec 16;35(50):16479-93.
Liu J, Liu Y, Barter RA, Klaassen CD.: Alteration of thyroid homeostasis by UDP-glucuronosyltransferase inducers in rats: a dose-response study. J Pharmacol Exp Ther 273, 977-85, 1994.
McClain RM. Mechanistic considerations for the relevance of animal data on thyroid neoplasia to human risk assessment. Mutat Res. 1995 Dec;333(1-2):131-42
Morreale de Escobar, G. The role of thyroid hormone in fetal neurodevelopment. J Pediat Endocrinol Metabol. 2001. 14; 1453-62.
Morse DC, Wehler EK, Wesseling W, Koeman JH, Brouwer A. Alterations in rat brain thyroid hormone status following pre- and postnatal exposure to polychlorinated biphenyls (Aroclor 1254). Toxicol Appl Pharmacol. 1996 Feb;136(2):269-79.
NTP National Toxicology Program.: NTP toxicology and carcinogenesis studies of 3,3'-dimethylbenzidine dihydrochloride (CAS no. 612-82-8) in F344/N rats (drinking water studies). Natl Toxicol Program Tech Rep Ser 390, 1-238, 1991.
O'Connor, J. C., J. C. Cook, et al. (1998). "An ongoing validation of a Tier I screening battery for detecting endocrine-active compounds (EACs)." Toxicol Sci 46(1): 45-60.
O'Connor, J. C., L. G. Davis, et al. (2000). "Detection of dopaminergic modulators in a tier I screening battery for identifying endocrine-active compounds (EACs)." Reprod Toxicol 14(3): 193-205.
Paul KB, Hedge JM, Rotroff DM, Hornung MW, Crofton KM, Simmons SO. Development of a thyroperoxidase inhibition assay for high-throughput screening. Chem Res Toxicol. 2014 Mar 17;27(3):387-99
Paul KB, Hedge JM, Macherla C, Filer DL, Burgess E, Simmons SO, Crofton KM, Hornung MW. Cross-species analysis of thyroperoxidase inhibition by xenobiotics demonstrates conservation of response between pig and rat. Toxicology. 2013. 312:97-107
Paul-Friedman K, Watt ED, Hornung MW, Hedge JM, Judson RS, Crofton KM, Houck KA, Simmons SO. 2016. Tiered High-Throughput Screening Approach to Identify Thyroperoxidase Inhibitors Within the ToxCast Phase I and II Chemical Libraries. Toxicol Sci. 151:160-80.
Schneider S, Kaufmann W, Strauss V, van Ravenzwaay B. Vinclozolin: a feasibility and sensitivity study of the ILSI-HESI F1-extended one-generation rat reproduction protocol. Regul Toxicol Pharmacol. 2011 Feb;59(1):91-100.
Sharlin DS, Gilbert ME, Taylor MA, Ferguson DC, Zoeller RT. The nature of the compensatory response to low thyroid hormone in the developing brain. J Neuroendocrinol. 2010 Mar;22(3):153-65. d
Spilker C, Nullmeier S, Grochowska KM, Schumacher A, Butnaru I, Macharadze T, Gomes GM, Yuanxiang P, Bayraktar G, Rodenstein C, Geiseler C, Kolodziej A, Lopez-Rojas J, Montag D, Angenstein F, Bär J, D'Hanis W, Roskoden T, MikhaylovaM, Budinger E, Ohl FW, Stork O, Zenclussen AC, Karpova A, Schwegler H, Kreutz MR.A Jacob/Nsmf Gene Knockout Results in Hippocampal Dysplasia and Impared BDNFSignaling in Dendritogenesis. PLoS Genet. 2016. 12(3):e1005907
Taurog A. Molecular evolution of thyroid peroxidase. Biochimie. 1999 May;81(5):557-62
Taurog A. 2005. Hormone synthesis. In: Werner and Ingbar’s The Thyroid: A Fundamental and Clinical Text (Braverman LE, Utiger RD, eds). Philadelphia:Lippincott, Williams and Wilkins, 47–81
US EPA (2011) FIFRA Scintific Advisory Panel Consultation. Integrated Approaches to Testing and Assessment Strategy: Use of New Computational and Molecular Tools, US. May 24, 26, 2011, US Environmental Protection Agency, Office of Pesticide Programs, Washington DC
Vickers AE, Heale J, Sinclair JR, Morris S, Rowe JM, Fisher RL Thyroid organotypic rat and human cultures used to investigate drug effects on thyroid function, hormone synthesis and release pathways. Toxicol Appl Pharmacol. 2012 260(1):81-8.
Zoeller RT, Crofton KM. Mode of action: developmental thyroid hormone insufficiency--neurological abnormalities resulting from exposure to propylthiouracil. Crit Rev Toxicol. 2005 35(8-9):771-81. Review. PubMed PMID: 16417044.
Zoeller, R. T., R. Bansal, et al. (2005). "Bisphenol-A, an environmental contaminant that acts as a thyroid hormone receptor antagonist in vitro, increases serum thyroxine, and alters RC3/neurogranin expression in the developing rat brain." Endocrinology 146(2): 607-612.
Zoeller RT, Rovet J. Timing of thyroid hormone action in the developing brain: clinical observations and experimental findings. J Neuroendocrinol. 2004 Oct;16(10):809-18