AOP ID and Title:
Graphical Representation
Status
| Author status | OECD status | OECD project | SAAOP status |
|---|---|---|---|
| Under development: Not open for comment. Do not cite |
Abstract
This AOP links in utero decreased intratesticular testosterone levels with hypospadias in male offspring. Hypospadias is a common reproductive disorder with a prevalence of up to ~1/125 newborn boys (Leunbach et al., 2025; Paulozzi, 1999). Developmental exposure to endocrine disrupting chemicals is suspected to contribute to some cases of hypospadias (Mattiske & Pask, 2021). Hypospadias can be indicative of fetal disruptions to male reproductive development, and is associated with short anogenital distance and cryptorchidism (Skakkebaek et al., 2016). Thus, hypospadias is included as an endpoint in OECD test guidelines (TG) for developmental and reproductive toxicity (TG 414, 416, 421/422, and 443; (OECD, 2001, 2016b, 2016a, 2018a, 2018b)), as both a measurement of adverse reproductive effects and a direct clinical adverse outcome.
Testosterone is one of the two main steroid sex hormones essential for male reproductive development. Testosterone is primarily, but not exclusively, produced in the testes and then secreted into the circulation. In peripheral reproductive tissues, testosterone is either converted to dihydrotestosterone (DHT) or directly activates the androgen receptor (AR). DHT is more potent than testosterone in activating the AR, and activation of AR by either androgen initiates differentiation of the male phenotype, including development of the penis (Amato et al., 2022; Davey & Grossmann, 2016). This AOP delineates the evidence that decreasing testicular testosterone production lowers circulating testosterone levels and consequently AR activation, thereby disrupting penis development and causing hypospadias. In this AOP, the first KE is not considered an MIE, as testicular testosterone production can be obstructed by various routes. The AOP does not discriminate whether the reduction in AR activation is due to direct lack of testosterone at the AR or due to decreased conversion of testosterone to DHT, as there is not sufficient information on this. The AOP is supported by in vitro experiments upstream of AR activation and by in vivo and human case studies downstream of AR activation. Downstream of a reduction in AR activation, the molecular mechanisms of hypospadias development are not fully delineated, highlighting a knowledge gap in this AOP. Thus, the AOP has potential for inclusion of additional KEs and elaboration of molecular causality links, once these are established. Given that hypospadias is both a clinical and toxicological endpoint, this AOP is considered highly relevant in a regulatory context.
Background
This AOP is a part of an AOP network for reduced androgen receptor activation causing hypospadias in male offspring. The other AOPs in this network are AOP-477 (‘Androgen receptor antagonism leading to hypospadias in male (mammalian) offspring’), and AOP-571 (‘5α-reductase inhibition leading to hypospadias in male (mammalian) offspring’). The purpose of the AOP network is to organize the well-established evidence for anti-androgenic mechanisms-of-action leading to hypospadias, thus informing predictive toxicology and identifying knowledge gaps for investigation and method development.
This work received funding from the European Food and Safety Authority (EFSA) under Grant agreement no. GP/EFSA/PREV/2022/01 and from the Danish Environmental Protection Agency under the Danish Center for Endocrine Disrupters (CeHoS).
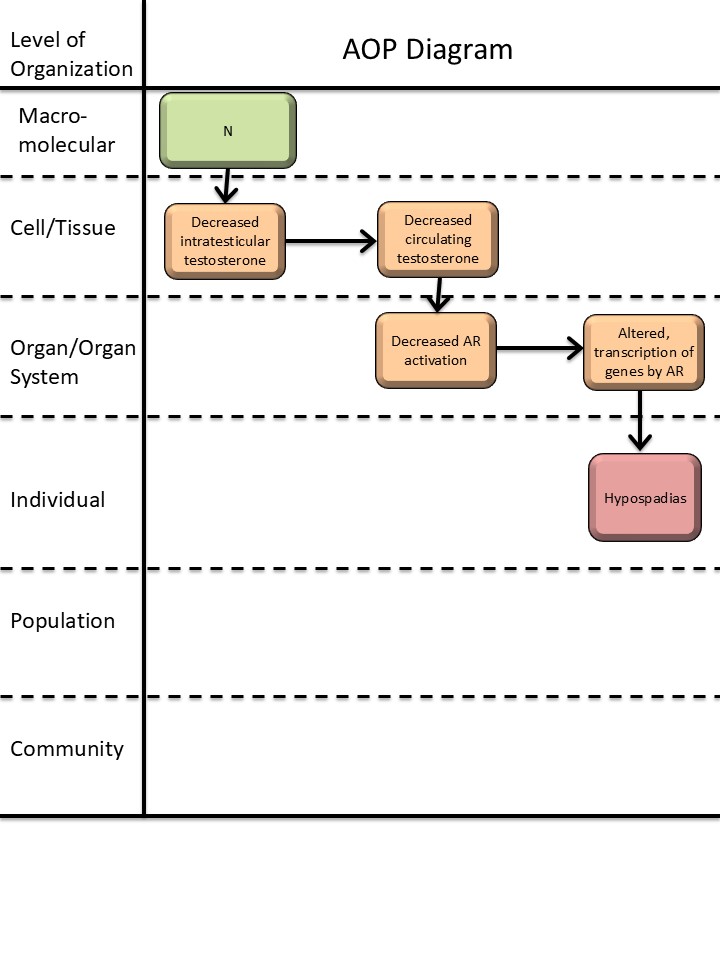
Summary of the AOP
Events
Molecular Initiating Events (MIE), Key Events (KE), Adverse Outcomes (AO)
| Sequence | Type | Event ID | Title | Short name |
|---|---|---|---|---|
| KE | 2298 | Decrease, intratesticular testosterone levels | Decrease, intratesticular testosterone | |
| KE | 1690 | Decrease, circulating testosterone levels | Decrease, circulating testosterone levels | |
| KE | 1614 | Decrease, androgen receptor activation | Decrease, AR activation | |
| KE | 286 | Altered, Transcription of genes by the androgen receptor | Altered, Transcription of genes by the AR | |
| AO | 2082 | Hypospadias, increased | Hypospadias |
Key Event Relationships
| Upstream Event | Relationship Type | Downstream Event | Evidence | Quantitative Understanding |
|---|---|---|---|---|
| Decrease, intratesticular testosterone levels | adjacent | Decrease, circulating testosterone levels | High | |
| Decrease, circulating testosterone levels | adjacent | Decrease, androgen receptor activation | High | |
| Decrease, androgen receptor activation | adjacent | Altered, Transcription of genes by the androgen receptor | High | |
| Decrease, intratesticular testosterone levels | non-adjacent | Hypospadias, increased | Moderate | |
| Decrease, circulating testosterone levels | non-adjacent | Hypospadias, increased | Low | |
| Decrease, androgen receptor activation | non-adjacent | Hypospadias, increased | High |
Stressors
| Name | Evidence |
|---|---|
| Dibutyl phthalate | |
| Di(2-ethylhexyl) phthalate |
Overall Assessment of the AOP
Domain of Applicability
Life Stage Applicability| Life Stage | Evidence |
|---|---|
| Foetal | High |
| Term | Scientific Term | Evidence | Links |
|---|---|---|---|
| human | Homo sapiens | High | NCBI |
| rat | Rattus norvegicus | High | NCBI |
| mouse | Mus musculus | Moderate | NCBI |
| Sex | Evidence |
|---|---|
| Male | High |
Although the upstream part of the AOPN has a broad applicability domain, the overall AOPN is considered only applicable to male mammals during fetal life, restricted by the applicability of KE-2298 (‘Decrease, intratesticular testosterone levels’) and KER-3488 (‘Decrease, intratesticular testosterone levels leads to hypospadias’), KER-3350 (‘Decrease, circulating testosterone levels leads to hypospadias’), and KER-2828 (‘Decrease AR activation leads to hypospadias’). By definition, testes are the primary sex organs in males, and the term hypospadias is mainly used for describing malformation of the male and not female external genitalia. The genital tubercle is programmed by androgens to differentiate into a penis in fetal life in the masculinization programming window, followed by the morphological differentiation (Welsh et al., 2008). In humans, hypospadias is diagnosed at birth and can also often be observed in rodents at this time point, although the rodent penis does not finish developing until a few weeks after birth (Baskin & Ebbers, 2006; Sinclair et al., 2017). The disruption to androgen programming leading to hypospadias thus happens in the fetal life stage, but the AO is best detected postnatally. Regarding taxonomic applicability, hypospadias has mainly been identified in rodents (rats and mice) and humans, and the evidence in this AOP is almost exclusively from these species. It is, however, biologically plausible that the AOP is applicable to other mammals as well, given the conserved role of androgens in mammalian reproductive development, and hypospadias has been observed in many domestic animal and wildlife species, albeit not coupled to reduced testosterone levels.
Essentiality of the Key Events
|
Event |
Evidence |
Uncertainties and inconsistencies |
|
KE-2298 Decrease, intratesticular testosterone levels (moderate) |
Biological plausibility provides strong support for the essentiality of this event as the testes are the main sites of testosterone production in male mammals, and testosterone is a ligand for the AR and one of the primary drivers of penis development.
Experimental evidence with phthalates lowering intratesticular testosterone supports the essentiality (see KE 3488)
Human case studies indirectly support the essentiality as mutations in steroidogenesis enzymes and gonadal dysgenesis are associated with low circulating testosterone levels and hypospadias (as listed in table 4, KER 2828) |
In the human studies, testosterone levels were only measured postnatally and not in fetal life. |
|
KE-1690 Decrease, circulating testosterone levels (moderate) |
Biological plausibility provides strong support for the essentiality of this event as testosterone is a ligand for the AR and one of the primary drivers of penis development
Human case studies support the essentiality as low circulating testosterone levels have been associated with hypospadias (as listed in table 4 in KER-2828). |
In human case studies, testosterone levels were only measured postnatally and not in fetal life. As hypospadias is a congenital malformation, it cannot be “reversed” by testosterone treatment. |
|
KE-1614 Decrease, AR activation (moderate) |
Biological plausibility provides strong support for the essentiality of this event, as AR activation is critical for normal penis development.
Conditional or full knockout of Ar in mice results in partly or full sex-reversal of males, including a female-like urethral opening (Willingham et al., 2006; Yucel et al., 2004; Zheng et al., 2015). Human subjects with AR mutations may also have associated hypospadias (as presented in table 4 in KER 2828). |
|
|
KE-286 Altered, transcription of genes by AR (low) |
Biological plausibility provides support for the essentiality of this event. AR is a nuclear receptor and transcription factor regulating transcription of genes, and androgens, acting through AR, are essential for normal male penis development. Known AR-responsive genes active in normal penis development have been thoroughly reviewed (Amato et al., 2022). |
There are currently no AR-responsive genes proved to be causally involved in hypospadias, and it is known that the AR can also signal through non-genomic actions (Leung & Sadar, 2017). |
|
Event |
Direct evidence |
Indirect evidence |
Contradictory evidence |
Overall essentiality assessment |
|
KE-2298 |
|
** |
* |
Moderate |
|
KE-1690 |
|
*** |
* |
Moderate |
|
KE-1614 |
** |
|
|
Moderate |
|
KE-286 |
|
* |
|
Low |
Weight of Evidence Summary
The confidence in each of the KERs comprising the AOP are judged as high, with both high biological plausibility and high confidence in the empirical evidence. The mechanistic link between KE-286 (‘altered, transcription of genes by AR’) and AO-2082 (‘hypospadias’) is not established, but given the high confidence in the KERs including the non-adjacent KER-2828 linking to the AO, the overall confidence in the AOP is judged as high.
|
KER |
Biological Plausibility |
Empirical Evidence |
Rationale |
|
KER-3448 Decrease, intratesticular testosterone levels leads to decrease, circulating testosterone levels |
High |
High (canonical) |
It is well established that testes are the primary testosterone-producing organs in male mammals. In vivo studies have shown that exposure to substances that lowers intratesticular testosterone also lowers circulating testosterone levels (Svingen et al., 2025). |
|
KER-2131 Decrease, circulating testosterone levels leads to decrease, AR activation |
High |
High (canonical) |
It is well established that testosterone activates the AR. Direct evidence for this KER is not possible since KE 1614 can currently not be measured and is considered an in vivo effect. Indirect evidence using proxy read-outs of AR activation, either in vitro or in vivo strongly supports the relationship (Draskau et al., 2024). |
|
KER-2124 Decrease, AR activation leads to altered, transcription of genes by AR |
High |
High (canonical) |
It is well established that the AR regulates gene transcription. In vivo animal studies and human genomic profiling show tissue-specific changes to gene expression upon disruption of AR (Draskau et al., 2024). |
|
KER-3488 Decrease, intratesticular testosterone leads to hypospadias |
High |
Moderate |
It is well established that testicular testosterone is one of the primary drivers of penis development. In vivo animal studies support that reductions in fetal testicular testosterone can cause hypospadias in male offspring. One study supports dose concordance, where diisocytol caused reduced ex vivo testosterone production in rats at a dose of 0.1 mg/kg bw/day, while hypospadias was observed in male offspring at 1 mg/kg bw/day (Saillenfait et al., 2013). |
|
KER-3350 Decrease, circulating testosterone levels leads to hypospadias |
High |
Low |
It is well established that testosterone is one of the primary drivers of penis development. In vivo evidence for this KER is sparse, but human case studies of subjects with low testosterone levels (postnatally) and associated hypospadias support the KER.
|
|
KER-2828 Decrease, AR activation leads to hypospadias |
High |
High |
It is well established that AR drives penis differentiation. Numerous in vivo toxicity studies and human case studies indirectly show that decreased AR activation leads to hypospadias, with few inconsistencies. The empirical evidence moderately supports dose, temporal, and incidence concordance for the KER. |
Quantitative Consideration
The quantitative understanding of this AOP is judged as low.
A model for phthalate-induced malformations has been developed which aims to predict the frequency of hypospadias related to a phthalate’s reduction in ex vivo testosterone production. The model predicted that a 60% reduction in testosterone levels would induce hypospadias, although the predictivity of this model was not good when tested for one phthalate (Earl Gray et al., 2024).
References
Amato, C. M., Yao, H. H.-C., & Zhao, F. (2022). One Tool for Many Jobs: Divergent and Conserved Actions of Androgen Signaling in Male Internal Reproductive Tract and External Genitalia. Frontiers in Endocrinology, 13, 910964. https://doi.org/10.3389/fendo.2022.910964
Baskin, L., & Ebbers, M. (2006). Hypospadias: Anatomy, etiology, and technique. Journal of Pediatric Surgery, 41(3), 463–472. https://doi.org/10.1016/j.jpedsurg.2005.11.059
Bhasin, S., Cunningham, G. R., Hayes, F. J., Matsumoto, A. M., Snyder, P. J., Swerdloff, R. S., & Montori, V. M. (2010). Testosterone Therapy in Men with Androgen Deficiency Syndromes: An Endocrine Society Clinical Practice Guideline. The Journal of Clinical Endocrinology & Metabolism, 95(6), 2536–2559. https://doi.org/10.1210/jc.2009-2354
Chamberlain, N. L., Driver, E. D., & Miesfeld, R. L. (1994). The length and location of CAG trinucleotide repeats in the androgen receptor N-terminal domain affect transactivation function. Nucleic Acids Research, 22(15), 3181–3186. https://doi.org/10.1093/nar/22.15.3181
Coviello, A. D., Bremner, W. J., Matsumoto, A. M., Herbst, K. L., Amory, J. K., Anawalt, B. D., Yan, X., Brown, T. R., Wright, W. W., Zirkin, B. R., & Jarow, J. P. (2004). Intratesticular Testosterone Concentrations Comparable With Serum Levels Are Not Sufficient to Maintain Normal Sperm Production in Men Receiving a Hormonal Contraceptive Regimen. Journal of Andrology, 25(6), 931–938. https://doi.org/10.1002/j.1939-4640.2004.tb03164.x
Davey, R. A., & Grossmann, M. (2016). Androgen Receptor Structure, Function and Biology: From Bench to Bedside. The Clinical Biochemist. Reviews, 37(1), 3–15.
Draskau, M., Rosenmai, A., Bouftas, N., Johansson, H., Panagiotou, E., Holmer, M., Elmelund, E., Zilliacus, J., Beronius, A., Damdimopoulou, P., van Duursen, M., & Svingen, T. (2024). Aop Report: An Upstream Network for Reduced Androgen Signalling Leading to Altered Gene Expression of Ar Responsive Genes in Target Tissues. Environ Toxicol Chem, In Press.
Earl Gray, L. J., Lambright, C. S., Evans, N., Ford, J., & Conley, J. M. (2024). Using targeted fetal rat testis genomic and endocrine alterations to predict the effects of a phthalate mixture on the male reproductive tract. Current Research in Toxicology, 7, 100180–100180. https://doi.org/10.1016/j.crtox.2024.100180
Holmer, M. L., Zilliacus, J., Draskau, M. K., Hlisníková, H., Beronius, A., & Svingen, T. (2024). Methodology for developing data-rich Key Event Relationships for Adverse Outcome Pathways exemplified by linking decreased androgen receptor activity with decreased anogenital distance. Reproductive Toxicology, 128, 108662. https://doi.org/10.1016/j.reprotox.2024.108662
Leunbach, T. L., Berglund, A., Ernst, A., Hvistendahl, G. M., Rawashdeh, Y. F., & Gravholt, C. H. (2025). Prevalence, Incidence, and Age at Diagnosis of Boys With Hypospadias: A Nationwide Population-Based Epidemiological Study. Journal of Urology, 213(3), 350–360. https://doi.org/10.1097/JU.0000000000004319
Leung, J. K., & Sadar, M. D. (2017). Non-Genomic Actions of the Androgen Receptor in Prostate Cancer. Frontiers in Endocrinology, 8. https://doi.org/10.3389/fendo.2017.00002
Mattiske, D. M., & Pask, A. J. (2021). Endocrine disrupting chemicals in the pathogenesis of hypospadias; developmental and toxicological perspectives. Current Research in Toxicology, 2, 179–191. https://doi.org/10.1016/j.crtox.2021.03.004
McLachlan, R. I., O’Donnell, L., Stanton, P. G., Balourdos, G., Frydenberg, M., de Kretser, D. M., & Robertson, D. M. (2002). Effects of Testosterone Plus Medroxyprogesterone Acetate on Semen Quality, Reproductive Hormones, and Germ Cell Populations in Normal Young Men. The Journal of Clinical Endocrinology & Metabolism, 87(2), 546–556. https://doi.org/10.1210/jcem.87.2.8231
OECD. (2001). Test No. 416: Two-Generation Reproduction Toxicity [OECD Guidelines for the Testing of Chemicals, Section 4]. OECD Publishing. https://doi.org/10.1787/9789264070868-en
OECD. (2016a). Test No. 421: Reproduction/Developmental Toxicity Screening Test. OECD. https://doi.org/10.1787/9789264264380-en
OECD. (2016b). Test No. 422: Combined Repeated Dose Toxicity Study with the Reproduction/Developmental Toxicity Screening Test. OECD. https://doi.org/10.1787/9789264264403-en
OECD. (2018a). Test No. 414: Prenatal Developmental Toxicity Study. OECD. https://doi.org/10.1787/9789264070820-en
OECD. (2018b). Test No. 443: Extended One-Generation Reproductive Toxicity Study. OECD. https://doi.org/10.1787/9789264185371-en
Paulozzi, L. J. (1999). International trends in rates of hypospadias and cryptorchidism.
Saillenfait, A., Sabaté, J., Robert, A., Cossec, B., Roudot, A., Denis, F., & Burgart, M. (2013). Adverse effects of diisooctyl phthalate on the male rat reproductive development following prenatal exposure. Reproductive Toxicology (Elmsford, N.Y.), 42, 192–202. https://doi.org/10.1016/j.reprotox.2013.09.004
Sinclair, A., Cao, M., Pask, A., Baskin, L., & Cunha, G. (2017). Flutamide-induced hypospadias in rats: A critical assessment. Differentiation; Research in Biological Diversity, 94, 37–57. https://doi.org/10.1016/j.diff.2016.12.001
Skakkebaek, N. E., Rajpert-De Meyts, E., Louis, G. M. B., Toppari, J., Andersson, A.-M., Eisenberg, M. L., Jensen, T. K., Jorgensen, N., Swan, S. H., Sapra, K. J., Ziebe, S., Priskorn, L., & Juul, A. (2016). Male Reproductive Disorders And Fertility Trends: Influences Of Environement And Genetic susceptibility. PHYSIOLOGICAL REVIEWS, 96(1), 55–97. https://doi.org/10.1152/physrev.00017.2015
Svingen, T., Elmelund, E., Holmer, M. L., Bindel, A. O., Holbech, H., & Draskau, M. K. (2025). AOP report: Adverse Outcome Pathway Network for Developmental Androgen Signalling-Inhibition Leading to Short Anogenital Distance in Male Offspring. Environmental Toxicology and Chemistry, vgaf221. https://doi.org/10.1093/etojnl/vgaf221
Svingen, T., Villeneuve, D. L., Knapen, D., Panagiotou, E. M., Draskau, M. K., Damdimopoulou, P., & O’Brien, J. M. (2021). A Pragmatic Approach to Adverse Outcome Pathway Development and Evaluation. Toxicological Sciences, 184(2), 183–190. https://doi.org/10.1093/toxsci/kfab113
Turner, T. T., Jones, C. E., Howards, S. S., Ewing, L. L., Zegeye, B., & Gunsalus, G. L. (1984). On the androgen microenvironment of maturing spermatozoa. Endocrinology, 115(5), 1925–1932. https://doi.org/10.1210/endo-115-5-1925
Tut, T. G., Ghadessy, F. J., Trifiro, M. A., Pinsky, L., & Yong, E. L. (1997). Long Polyglutamine Tracts in the Androgen Receptor Are Associated with Reduced Trans -Activation, Impaired Sperm Production, and Male Infertility 1. The Journal of Clinical Endocrinology & Metabolism, 82(11), 3777–3782. https://doi.org/10.1210/jcem.82.11.4385
Welsh, M., Saunders, P. T. K., Fisken, M., Scott, H. M., Hutchison, G. R., Smith, L. B., & Sharpe, R. M. (2008). Identification in rats of a programming window for reproductive tract masculinization, disruption of which leads to hypospadias and cryptorchidism. Journal of Clinical Investigation, 118(4), 1479–1490. https://doi.org/10.1172/JCI34241
Willingham, E., Agras, K., Souza, A. J. de, Konijeti, R., Yucel, S., Rickie, W., Cunha, G., & Baskin, L. (2006). Steroid receptors and mammalian penile development: An unexpected role for progesterone receptor? The Journal of Urology, 176(2), 728–733. https://doi.org/10.1016/j.juro.2006.03.078
Yucel, S., Liu, W., Cordero, D., Donjacour, A., Cunha, G., & Baskin, L. (2004). Anatomical studies of the fibroblast growth factor-10 mutant, Sonic Hedge Hog mutant and androgen receptor mutant mouse genital tubercle. Advances in Experimental Medicine and Biology, 545, 123–148. https://doi.org/10.1007/978-1-4419-8995-6_8
Zheng, Z., Armfield, B., & Cohn, M. (2015). Timing of androgen receptor disruption and estrogen exposure underlies a spectrum of congenital penile anomalies. Proceedings of the National Academy of Sciences of the United States of America, 112(52), E7194-203. https://doi.org/10.1073/pnas.1515981112
Appendix 1
List of Key Events in the AOP
Event: 2298: Decrease, intratesticular testosterone levels
Short Name: Decrease, intratesticular testosterone
Key Event Component
| Process | Object | Action |
|---|---|---|
| testosterone biosynthetic process | testosterone | decreased |
AOPs Including This Key Event
Biological Context
| Level of Biological Organization |
|---|
| Organ |
Organ term
| Organ term |
|---|
| testis |
Domain of Applicability
Taxonomic Applicability Life Stage Applicability| Life Stage | Evidence |
|---|---|
| During development and at adulthood | High |
| Sex | Evidence |
|---|---|
| Male | High |
This key event (KE) is applicable to all male vertebrates with testis that produce testosterone.
Key Event Description
This KE refers to decreased testosterone biosynthesis in the testis (male); i.e. intratesticular testosterone levels. It is therefore considered distinct from KEs describing circulating testosterone levels, or levels in any other tissue or organ of vertebrate animals. It is also distinct from indirect cell-based assays measuring effects on testosterone synthesis, including in vitro Leydig cells.
In males, the testis is the primary site of testosterone biosynthesis via the steroidogenesis pathway – an enzymatic pathway converting cholesterol into all the downstream steroid hormones (Miller and Auchus 2010). In mammals, the Leydig cells are considered the primary site of steroidogenesis in the testis. Although generally correct, there is evidence to suggest the involvement of Sertoli cells during fetal stages in e.g. mouse and human testis, but with Leydig cells being sufficient in adult life (O’Donnell et al 2022).
Testicular testosterone synthesis is primarily regulated by the hypothalamic-pituitary-gonadal (HPG) axis, with Gonadotropin-releasing hormone (GnRH) from the hypothalamus controlling the secretion of Luteinizing hormone (LH) from the pituitary that ultimately binds to the LH receptors on Leydig cells to stimulate steroidogenesis. Notably, the timing of HPG axis activation during development varies between species. In humans, human chorionic gonadotropin (hCG) act similarly to LH and appear to be critical in stimulating testosterone synthesis in the fetal testis (Huhtaniemi 2025), whereas in the mouse testosterone synthesis in the fetal testis appears to be independent of pituitary gonadotropins even though LH is detectable during late gestation O’Shaughnessy et al 1998). Irrespective of testosterone being stimulated by gonadotropins or occurring de novo, however, it is essential for masculinization of the developing fetus, initiation of puberty, and maintain reproductive, and other, functions in adulthood.
Notably, intratesticular testosterone concentration is significantly higher than serum testosterone levels, typically ranging from 30- to 200-fold greater in mammals, including humans (Turner et al 1984; McLachlan et al 2002; Coviello et al 2004).
How it is Measured or Detected
Testosterone levels can be quantified in testis tissue (ex vivo, in vivo). Methods include traditional immunoassays such as ELISA and RIA, advanced techniques like LC-MS/MS, and liquid scintillation spectrometry following radiolabeling (Shiraishi et al., 2008).
References
Coviello, A.D., Bremner, W.J., Matsumoto, A.M., Herbst, K.L., Amory, J.K., Anawalt, B.D., Yan, X., Brown, T.R., Wright, W.W., Zirkin, B.R. and Jarow, J.P. (2004). Intratesticular Testosterone Concentrations Comparable With Serum Levels Are Not Sufficient to Maintain Normal Sperm Production in Men Receiving a Hormonal Contraceptive Regimen. J Androl, 25:931-938. https://doi.org/10.1002/j.1939-4640.2004.tb03164.x
Huhtaniemi, I.T. (2025). Luteinizing hormone receptor knockout mouse: What has it taught us? Andrology, In Press. https://doi.org/10.1111/andr.70000
McLachlan, R.I., O’Donnell, L., Stanton, P.G., Balourdos, G., Frydenberg, M., de Kretser, D.M. and Robertson, D.M. (2002). Effects of testosterone plus medroxyprogesterone acetate on semen quality, reproductive hormones, and germ cell populations in normal young men. J Clin Endocriol Metab, 87:546-556. https://doi.org/10.1210/jcem.87.2.8231
Miller, W.L. and Auchus, R.J. (2010). The Molecular Biology, Biochemistry, and Physiology of Human Steroidogenesis and Its Disorders. Endocr Rev, 32(1):81-151. https://doi.org/10.1210/er.2010-0013
O’Donnell, L., Whiley, P.A.F., and Loveland, K.L. (2022). Activin A and Sertoli Cells: Key to Fetal Testis Steroidogenesis. Front Endocrinol, 13:898876. https://doi.org/10.3389/fendo.2022.898876
O’Shaughnessy, P.J., Baker, P., Sohnius, U., Haavisto, A.M., Charlton, H.M. and Huhtaniemi, I. (1998). Fetal development of Leydig cell activity in the mouse is independent of pituitary gonadotroph function. Endocrinology, 139:1141-1146. https://doi.org/10.1210/endo.139.3.5788
Shiraishi, S., Lee, P. W. N., Leung, A., Goh, V. H. H., Swerdloff, R. S., & Wang, C. (2008). Simultaneous Measurement of Serum Testosterone and Dihydrotestosterone by Liquid Chromatography–Tandem Mass Spectrometry. Clinical Chemistry, 54(11), 1855–1863. https://doi.org/10.1373/clinchem.2008.103846
Turner, T.T., Jones, C.E., Howards, S.S., Ewing, L.L., Zegeye, B. and Gunsalus, G.L. (1984). On the androgen microenvironment of maturing spermatozoa. Endocrinology, 115:1925-1932. https://doi.org/10.1210/endo-115-5-1925
Event: 1690: Decrease, circulating testosterone levels
Short Name: Decrease, circulating testosterone levels
Key Event Component
| Process | Object | Action |
|---|---|---|
| hormone biosynthetic process | testosterone | decreased |
| testosterone biosynthetic process | testosterone | decreased |
AOPs Including This Key Event
Biological Context
| Level of Biological Organization |
|---|
| Tissue |
Organ term
| Organ term |
|---|
| blood |
Domain of Applicability
Taxonomic Applicability| Term | Scientific Term | Evidence | Links |
|---|---|---|---|
| mammals | mammals | High | NCBI |
| Life Stage | Evidence |
|---|---|
| During development and at adulthood | High |
| Sex | Evidence |
|---|---|
| Male | High |
| Female | High |
This key event (KE) is applicable to all mammals, as the synthesis and role of testosterone are evolutionarily conserved (Vitousek et al., 2018). Both sexes produce and require testosterone, which plays critical roles throughout life, from development to adulthood; albeit there are differences in lief stages when testosterone exert specific effects and function (Luetjens & Weinbauer, 2012; Naamneh Elzenaty et al., 2022). Accordingly, this KE applies to both males and females across all life stages, but life stage should be considered when embedding in AOPs.
Notably, the key enzymes involved in testosterone production first appeared in the common ancestor of amphioxus and vertebrates (Baker, 2011). This suggests that the KE has a broader domain of applicability, encompassing non-mammalian vertebrates. AOP developers are encouraged to integrate additional knowledge to expand its relevance beyond mammals to other vertebrates.
Key Event Description
Testosterone is an endogenous steroid hormone that acts by binding the androgen receptor (AR) in androgen-responsive tissues (Murashima et al., 2015). As with all steroid hormones, testosterone is produced through steroidogenesis, an enzymatic pathway converting cholesterol into all the downstream steroid hormones. Briefly, androstenedione or androstenediol is converted to testosterone by the enzymes 17β-hydroxysteroid dehydrogenase (HSD) or 3β-HSD, respectively. Testosterone can then be converted to the more potent androgen, dihydrotestosterone (DHT) by 5α-reductase, or aromatized by CYP19A1 (Aromatase) into estrogens. Testosterone secreted in blood circulation can be found free or bound to SHBG or albumin (Trost & Mulhall, 2016).
Testosterone is produced mainly by the testes (in males), ovaries (in females) and to a lesser degree in the adrenal glands. The output of testosterone from different tissues varies with life stages. During fetal development testosterone is crucial for the differentiation of male reproductive tissues and the overall male phenotype. In adulthood, testosterone synthesis is controlled by the Hypothalamus-Pituitary-Gonadal (HPG) axis. GnRH is released from the hypothalamus inducing LH pulses secreted by the anterior pituitary. This LH surge leads to increased testosterone production, both in testes (males) and ovaries (females). If testosterone reaches low levels, this axis is once again stimulated to increase testosterone synthesis. This feedback loop is essential for maintenance of appropriate testosterone levels (Chandrashekar & Bartke, 1998; Ellis et al., 1983; Rey, 2021).
By disrupting e.g. steroidogenesis or the HPG-axis, testosterone synthesis or homeostasis may be disrupted and can lead to less testosterone being synthesized and released into circulation.
General role in biology
Androgens are essential hormones responsible for the development of the male phenotype during fetal life and for sexual maturation at puberty. In adulthood, androgens remain essential for the maintenance of male reproductive function and behavior but is also essential for female fertility. Apart from their effects on reproduction, androgens affect a wide variety of non-reproductive tissues such as skin, bone, muscle, and brain (Heemers et al 2006). Androgens, principally testosterone and DHT, exert most of their effects by interacting with the AR (Murashima et al 2015).
How it is Measured or Detected
Testosterone levels can be quantified in serum (in vivo), cell culture medium (in vitro), or tissue (ex vivo, in vitro). Methods include traditional immunoassays such as ELISA and RIA, advanced techniques like LC-MS/MS, and liquid scintillation spectrometry following radiolabeling (Shiraishi et al., 2008).
The H295R Steroidogenesis Assay (OECD TG 456) is (currently; anno 2025) primarily used to measure estradiol and testosterone production. This validated OECD test guideline uses adrenal H295R cells, with hormone levels measured in the cell culture medium (OECD, 2011). H295R adrenocortical carcinoma cells express the key enzymes and hormones of the steroidogenic pathway, enabling broad analysis of steroidogenesis disruption by quantifying hormones in the medium using LC-MS/MS. Initially designed to assess testosterone and estradiol levels, the assay now extends to additional steroid hormones, such as progesterone and pregnenolone. The U.S. EPA’s ToxCast program further advanced this method, enabling high-throughput measurement of 11 steroidogenesis-related hormones (Haggard et al., 2018). While the H295R assay indirectly reflects disruptions in overall steroidogenesis (e.g., changes in testosterone levels), it does not provide mechanistic insights.
Testosterone can be measured by immunoassays and by isotope-dilution gas chromatography-mass spectrometry in serum (Taieb et al., 2003; Paduch et al., 2014). Testosterone levels may also be measured by: Fish Lifecycle Toxicity Test (FLCTT) (US EPA OPPTS 850.1500), Male pubertal assay (PP Male Assay) (US EPA OPPTS 890.1500), OECD TG 441: Hershberger Bioassay in Rats (H Assay).
References
Baker, M.E. (2011). Insights from the structure of estrogen receptor into the evolution of estrogens: implications for endocrine disruption. Biochem Pharmacol, 82(1), 1-8. https://doi.org/10.1016/j.bcp.2011.03.008
Chandrashekar, V., & Bartke, A. (1998). The Role of Growth Hormone in the Control of Gonadotropin Secretion in Adult Male Rats*. Endocrinology, 139(3), 1067–1074. https://doi.org/10.1210/endo.139.3.5816
Ellis, G. B., Desjardins, C., & Fraser, H. M. (1983). Control of Pulsatile LH Release in Male Rats. Neuroendocrinology, 37(3), 177–183. https://doi.org/10.1159/000123540
Haggard, D. E., Karmaus, A. L., Martin, M. T., Judson, R. S., Setzer, R. W., & Paul Friedman, K. (2018). High-Throughput H295R Steroidogenesis Assay: Utility as an Alternative and a Statistical Approach to Characterize Effects on Steroidogenesis. Toxicological Sciences, 162(2), 509–534. https://doi.org/10.1093/toxsci/kfx274
Heemers, H. V, Verhoeven, G., & Swinnen, J. V. (2006). Androgen activation of the sterol regulatory element-binding protein pathway: Current insights. Molecular Endocrinology (Baltimore, Md.), 20(10), 2265–77. doi:10.1210/me.2005-0479
Luetjens, C. M., & Weinbauer, G. F. (2012). Testosterone: biosynthesis, transport, metabolism and (non-genomic) actions. In Testosterone (pp. 15–32). Cambridge University Press. https://doi.org/10.1017/CBO9781139003353.003
Murashima, A., Kishigami, S., Thomson, A., & Yamada, G. (2015). Androgens and mammalian male reproductive tract development. Biochimica et Biophysica Acta (BBA) - Gene Regulatory Mechanisms, 1849(2), 163–170. https://doi.org/10.1016/j.bbagrm.2014.05.020
Naamneh Elzenaty, R., du Toit, T., & Flück, C. E. (2022). Basics of androgen synthesis and action. Best Practice & Research Clinical Endocrinology & Metabolism, 36(4), 101665. https://doi.org/10.1016/j.beem.2022.101665
Paduch, D. A., Brannigan, R. E., Fuchs, E. F., Kim, E. D., Marmar, J. L., & Sandlow, J. I. (2014). The laboratory diagnosis of testosterone deficiency. Urology, 83(5), 980–8. https://doi.org/10.1016/j.urology.2013.12.024
Rey, R. A. (2021). The Role of Androgen Signaling in Male Sexual Development at Puberty. Endocrinology, 162(2). https://doi.org/10.1210/endocr/bqaa215
Shiraishi, S., Lee, P. W. N., Leung, A., Goh, V. H. H., Swerdloff, R. S., & Wang, C. (2008). Simultaneous Measurement of Serum Testosterone and Dihydrotestosterone by Liquid Chromatography–Tandem Mass Spectrometry. Clinical Chemistry, 54(11), 1855–1863. https://doi.org/10.1373/clinchem.2008.103846
Taieb, J., Mathian, B., Millot, F., Patricot, M.-C., Mathieu, E., Queyrel, N., … Boudou, P. (2003). Testosterone measured by 10 immunoassays and by isotope-dilution gas chromatography-mass spectrometry in sera from 116 men, women, and children. Clinical Chemistry, 49(8), 1381–95.
Trost, L. W., & Mulhall, J. P. (2016). Challenges in Testosterone Measurement, Data Interpretation, and Methodological Appraisal of Interventional Trials. The Journal of Sexual Medicine, 13(7), 1029–1046. https://doi.org/10.1016/j.jsxm.2016.04.068
Vitousek, M. N., Johnson, M. A., Donald, J. W., Francis, C. D., Fuxjager, M. J., Goymann, W., Hau, M., Husak, J. F., Kircher, B. K., Knapp, R., Martin, L. B., Miller, E. T., Schoenle, L. A., Uehling, J. J., & Williams, T. D. (2018). HormoneBase, a population-level database of steroid hormone levels across vertebrates. Scientific Data, 5(1), 180097. https://doi.org/10.1038/sdata.2018.97
Event: 1614: Decrease, androgen receptor activation
Short Name: Decrease, AR activation
Key Event Component
| Process | Object | Action |
|---|---|---|
| androgen receptor activity | androgen receptor | decreased |
AOPs Including This Key Event
Biological Context
| Level of Biological Organization |
|---|
| Tissue |
Domain of Applicability
Taxonomic Applicability| Term | Scientific Term | Evidence | Links |
|---|---|---|---|
| mammals | mammals | High | NCBI |
| Life Stage | Evidence |
|---|---|
| During development and at adulthood | High |
| Sex | Evidence |
|---|---|
| Mixed | High |
This KE is considered broadly applicable across mammalian taxa as all mammals express the AR in numerous cells and tissues where it regulates gene transcription required for developmental processes and functions. It is, however, acknowledged that this KE most likely has a much broader domain of applicability extending to non-mammalian vertebrates. AOP developers are encouraged to add additional relevant knowledge to expand on the applicability to also include other vertebrates.
Key Event Description
This KE refers to decreased activation of the androgen receptor (AR) as occurring in complex biological systems such as tissues and organs in vivo. It is thus considered distinct from KEs describing either blocking of AR or decreased androgen synthesis.
The AR is a nuclear transcription factor with canonical AR activation regulated by the binding of the androgens such as testosterone or dihydrotestosterone (DHT). Thus, AR activity can be decreased by reduced levels of steroidal ligands (testosterone, DHT) or the presence of compounds interfering with ligand binding to the receptor (Davey & Grossmann, 2016; Gao et al., 2005).
In the inactive state, AR is sequestered in the cytoplasm of cells by molecular chaperones. In the classical (genomic) AR signaling pathway, AR activation causes dissociation of the chaperones, AR dimerization and translocation to the nucleus to modulate gene expression. AR binds to the androgen response element (ARE) (Davey & Grossmann, 2016; Gao et al., 2005). Notably, for transcriptional regulation the AR is closely associated with other co-factors that may differ between cells, tissues and life stages. In this way, the functional consequence of AR activation is cell- and tissue-specific. This dependency on co-factors such as the SRC proteins also means that stressors affecting recruitment of co-activators to AR can result in decreased AR activity (Heinlein & Chang, 2002).
Ligand-bound AR may also associate with cytoplasmic and membrane-bound proteins to initiate cytoplasmic signaling pathways with other functions than the nuclear pathway. Non-genomic AR signaling includes association with Src kinase to activate MAPK/ERK signaling and activation of the PI3K/Akt pathway. Decreased AR activity may therefore be a decrease in the genomic and/or non-genomic AR signaling pathways (Leung & Sadar, 2017).
How it is Measured or Detected
This KE specifically focuses on decreased in vivo activation, with most methods that can be used to measure AR activity carried out in vitro. They provide indirect information about the KE and are described in lower tier MIE/KEs (see for example MIE/KE-26 for AR antagonism, KE-1690 for decreased T levels and KE-1613 for decreased dihydrotestosterone levels). Assays may in the future be developed to measure AR activation in mammalian organisms.
References
Davey, R. A., & Grossmann, M. (2016). Androgen Receptor Structure, Function and Biology: From Bench to Bedside. The Clinical Biochemist. Reviews, 37(1), 3–15.
Gao, W., Bohl, C. E., & Dalton, J. T. (2005). Chemistry and structural biology of androgen receptor. Chemical Reviews, 105(9), 3352–3370. https://doi.org/10.1021/cr020456u
Heinlein, C. A., & Chang, C. (2002). Androgen Receptor (AR) Coregulators: An Overview. https://academic.oup.com/edrv/article/23/2/175/2424160
Leung, J. K., & Sadar, M. D. (2017). Non-Genomic Actions of the Androgen Receptor in Prostate Cancer. Frontiers in Endocrinology, 8. https://doi.org/10.3389/fendo.2017.00002
OECD (2022). Test No. 251: Rapid Androgen Disruption Activity Reporter (RADAR) assay. Paris: OECD Publishing doi:10.1787/da264d82-en.
|
|
|
Event: 286: Altered, Transcription of genes by the androgen receptor
Short Name: Altered, Transcription of genes by the AR
Key Event Component
| Process | Object | Action |
|---|---|---|
| regulation of gene expression | androgen receptor | decreased |
AOPs Including This Key Event
Stressors
| Name |
|---|
| Bicalutamide |
| Cyproterone acetate |
| Epoxiconazole |
| Flutamide |
| Flusilazole |
| Prochloraz |
| Propiconazole |
| Stressor:286 Tebuconazole |
| Triticonazole |
| Vinclozalin |
Biological Context
| Level of Biological Organization |
|---|
| Tissue |
Domain of Applicability
Taxonomic Applicability| Term | Scientific Term | Evidence | Links |
|---|---|---|---|
| mammals | mammals | High | NCBI |
| Life Stage | Evidence |
|---|---|
| During development and at adulthood | High |
| Sex | Evidence |
|---|---|
| Mixed | High |
Both the DNA-binding and ligand-binding domains of the AR are highly evolutionary conserved, whereas the transactivation domain show more divergence, which may affect AR-mediated gene regulation across species (Davey and Grossmann 2016). Despite certain inter-species differences, AR function mediated through gene expression is highly conserved, with mutation studies from both humans and rodents showing strong correlation for AR-dependent development and function (Walters et al. 2010).
This KE is considered broadly applicable across mammalian taxa, sex and developmental stages, as all mammals express the AR in numerous cells and tissues where it regulates gene transcription required for developmental processes and function. It is, however, acknowledged that this KE most likely has a much broader domain of applicability extending to non-mammalian vertebrates. AOP developers are encouraged to add additional relevant knowledge to expand on the applicability to also include other vertebrates.
Key Event Description
This KE refers to transcription of genes by the androgen receptor (AR) as occurring in complex biological systems such as tissues and organs in vivo. Rather than measuring individual genes, this KE aims to capture patterns of effects at transcriptome level in specific target cells/tissues. In other words, it can be replaced by specific KEs for individual adverse outcomes as information becomes available, for example the transcriptional toxicity response in prostate tissue for AO: prostate cancer, perineum tissue for AO: reduced AGD, etc. AR regulates many genes that differ between tissues and life stages and, importantly, different gene transcripts within individual cells can go in either direction since AR can act as both transcriptional activator and suppressor. Thus, the ‘directionality’ of the KE cannot be either reduced or increased, but instead describe an altered transcriptome.
The Androgen Receptor and its function
The AR belongs to the steroid hormone nuclear receptor family. It is a ligand-activated transcription factor with three domains: the N-terminal domain, the DNA-binding domain, and the ligand-binding domain with the latter being the most evolutionary conserved (Davey and Grossmann 2016). Androgens (such as dihydrotestosterone and testosterone) are AR ligands and act by binding to the AR in androgen-responsive tissues (Davey and Grossmann 2016). Human AR mutations and mouse knockout models have established a fundamental role for AR in masculinization and spermatogenesis (Maclean et al.; Walters et al. 2010; Rana et al. 2014). The AR is also expressed in many other tissues such as bone, muscles, ovaries and within the immune system (Rana et al. 2014).
Altered transcription of genes by the AR as a Key Event
Upon activation by ligand-binding, the AR translocates from the cytoplasm to the cell nucleus, dimerizes, binds to androgen response elements in the DNA to modulate gene transcription (Davey and Grossmann 2016). The transcriptional targets vary between cells and tissues, as well as with developmental stages and is also dependent on available co-regulators (Bevan and Parker 1999; Heemers and Tindall 2007). It should also be mentioned that the AR can work in other ‘non-canonial’ ways such as non-genomic signaling, and ligand-independent activation (Davey & Grossmann, 2016; Estrada et al, 2003; Jin et al, 2013).
A large number of known, and proposed, target genes of AR canonical signaling have been identified by analysis of gene expression following treatments with AR agonists (Bolton et al. 2007; Ngan et al. 2009, Jin et al. 2013).
How it is Measured or Detected
Altered transcription of genes by the AR can be measured by measuring the transcription level of known downstream target genes by RT-qPCR or other transcription analyses approaches, e.g. transcriptomics.
Since this KE aims to capture AR-mediated transcriptional patterns of effect, downstream bioinformatics analyses will typically be required to identify and compare effect footprints. Clusters of genes can be statistically associated with, for example, biological process terms or gene ontology terms relevant for AR-mediated signaling. Large transcriptomics data repositories can be used to compare transcriptional patterns between chemicals, tissues, and species (e.g. TOXsIgN (Darde et al, 2018a; Darde et al, 2018b), comparisons can be made to identified sets of AR ‘biomarker’ genes (e.g. as done in (Rooney et al, 2018)), and various methods can be used e.g. connectivity mapping (Keenan et al, 2019).
References
Bevan C, Parker M (1999) The role of coactivators in steroid hormone action. Exp. Cell Res. 253:349–356
Bolton EC, So AY, Chaivorapol C, et al (2007) Cell- and gene-specific regulation of primary target genes by the androgen receptor. Genes Dev 21:2005–2017. doi: 10.1101/gad.1564207
Darde, T. A., Gaudriault, P., Beranger, R., Lancien, C., Caillarec-Joly, A., Sallou, O., et al. (2018a). TOXsIgN: a cross-species repository for toxicogenomic signatures. Bioinformatics 34, 2116–2122. doi:10.1093/bioinformatics/bty040.
Darde, T. A., Chalmel, F., and Svingen, T. (2018b). Exploiting advances in transcriptomics to improve on human-relevant toxicology. Curr. Opin. Toxicol. 11–12, 43–50. doi:10.1016/j.cotox.2019.02.001.
Davey RA, Grossmann M (2016) Androgen Receptor Structure, Function and Biology: From Bench to Bedside. Clin Biochem Rev 37:3–15
Estrada M, Espinosa A, Müller M, Jaimovich E (2003) Testosterone Stimulates Intracellular Calcium Release and Mitogen-Activated Protein Kinases Via a G Protein-Coupled Receptor in Skeletal Muscle Cells. Endocrinology 144:3586–3597. doi: 10.1210/en.2002-0164
Heemers H V., Tindall DJ (2007) Androgen receptor (AR) coregulators: A diversity of functions converging on and regulating the AR transcriptional complex. Endocr. Rev. 28:778–808
Jin, Hong Jian, Jung Kim, and Jindan Yu. 2013. “Androgen Receptor Genomic Regulation.” Translational Andrology and Urology 2(3):158–77. doi: 10.3978/j.issn.2223-4683.2013.09.01
Keenan, A. B., Wojciechowicz, M. L., Wang, Z., Jagodnik, K. M., Jenkins, S. L., Lachmann, A., et al. (2019). Connectivity Mapping: Methods and Applications. Annu. Rev. Biomed. Data Sci. 2, 69–92. doi:10.1146/ANNUREV-BIODATASCI-072018-021211.
Maclean HE, Chu S, Warne GL, Zajact JD Related Individuals with Different Androgen Receptor Gene Deletions
MacLeod DJ, Sharpe RM, Welsh M, et al (2010) Androgen action in the masculinization programming window and development of male reproductive organs. In: International Journal of Andrology. Blackwell Publishing Ltd, pp 279–287
Ngan S, Stronach EA, Photiou A, et al (2009) Microarray coupled to quantitative RT–PCR analysis of androgen-regulated genes in human LNCaP prostate cancer cells. Oncogene 28:2051–2063. doi: 10.1038/onc.2009.68
Rana K, Davey RA, Zajac JD (2014) Human androgen deficiency: Insights gained from androgen receptor knockout mouse models. Asian J. Androl. 16:169–177
Rooney, J. P., Chorley, B., Kleinstreuer, N., and Corton, J. C. (2018). Identification of Androgen Receptor Modulators in a Prostate Cancer Cell Line Microarray Compendium. Toxicol. Sci. 166, 146–162. doi:10.1093/TOXSCI/KFY187.
Walters KA, Simanainen U, Handelsman DJ (2010) Molecular insights into androgen actions in male and female reproductive function from androgen receptor knockout models. Hum Reprod Update 16:543–558. doi: 10.1093/humupd/dmq003
List of Adverse Outcomes in this AOP
Event: 2082: Hypospadias, increased
Short Name: Hypospadias
Key Event Component
| Process | Object | Action |
|---|---|---|
| embryonic organ development | penis | abnormal |
AOPs Including This Key Event
Biological Context
| Level of Biological Organization |
|---|
| Organ |
Organ term
| Organ term |
|---|
| penis |
Domain of Applicability
Taxonomic Applicability| Term | Scientific Term | Evidence | Links |
|---|---|---|---|
| human | Homo sapiens | High | NCBI |
| mouse | Mus musculus | High | NCBI |
| rat | Rattus norvegicus | High | NCBI |
| mammals | mammals | NCBI |
| Life Stage | Evidence |
|---|---|
| Perinatal | High |
| Sex | Evidence |
|---|---|
| Male | High |
Taxonomic applicability: Numerous studies have shown an association in humans between in utero exposure to endocrine disrupting chemicals and hypospadias. In mice and rats, in utero exposure to several endocrine disrupting chemicals, in particular estrogens and antiandrogens, have been shown to cause hypospadias in male offspring at different frequencies (Mattiske & Pask, 2021). Androgen-driven development of the male external genitalia is evolutionary conserved in most mammals and, to some extent, also in other vertebrate classes (Gredler et al., 2014). Hypospadias can in principle occur in all animals that form a genital tubercle and have been observed in many domestic animal species and wildlife species.
Life stage applicability: Penis development is finished prenatally in humans, and hypospadias is diagnosed at birth (Baskin & Ebbers, 2006). In rodents, penis development is not fully completed until weeks after birth, but hypospadias may be identified in early postnatal life as well, and in some cases in late gestation (Sinclair et al., 2017).
Sex applicability: Hypospadias is primarily used in reference to malformation of the male external genitalia.
Key Event Description
Hypospadias is a malformation of the penis where the urethral opening is displaced from the tip of the glans, usually on the ventral side on the phallus. Most cases of hypospadias are milder where the urethral opening still appears on the glans proper or on the most distal part of the shaft. In more severe cases, the opening may be more proximally placed on the shaft or even as low as the scrotum or the perineum.
In addition to the misplacement of the urethral opening, hypospadias is associated with an absence of ventral prepuce, an excess of dorsal preputial tissue, and in some cases a downward curvature of the penis (chordee). Patients with hypospadias may need surgical repairment depending on severity, with more proximal hypospadias patients in most need of surgeries to achieve optimal functional and cosmetic results (Baskin, 2000; Baskin & Ebbers, 2006; Mattiske & Pask, 2021). The incidence of hypospadias varies greatly between countries, from 1:100 to 1:500 of newborn boys (Skakkebaek et al., 2016), and the global prevalence seems to be increasing (Paulozzi, 1999; Springer et al., 2016; Yu et al., 2019).
The external genitalia arise from the biphasic genital tubercle during fetal development. Androgens (testosterone and dihydrotestosterone) drive formation of the male external genitalia. In humans, the urethra develops by fusion of two endoderm-derived urethral folds. Disruption of genital tubercle differentiation results in an incomplete urethra, i.e. hypospadias. (Baskin, 2000; Baskin & Ebbers, 2006).
How it is Measured or Detected
In humans, hypospadias is diagnosed clinically by physical examination of the infant and is at first recognized by the absence of ventral prepuce and concurrent excess dorsal prepuce (Baskin, 2000). Hypospadias may be classified according to the location of the urethral meatus: Glandular, subcoronal, midshaft, penoscrotal, scrotal, and perineal (Baskin & Ebbers, 2006).
In mice and rats, macroscopic assessment of hypospadias may be performed postnatally, and several OECD test guidelines require macroscopic examination of genital abnormalities in in vivo toxicity studies (TG 414, 416, 421/422, 443). The guidelines do not define hypospadias or how to identify them. Fetal and neonatal identification of hypospadias may require microscopic examination for proper evaluation of the pathology. This can be done by scanning electron microscopy (Uda et al., 2004), or by histological assessment in which the presence of the urethral opening in proximal, transverse sections (for example co-occuring with the os penis or corpus cavernosum), indicates hypospadias (Mahawong et al., 2014; Sinclair et al., 2017; Vilela et al., 2007). In a semiquantitative, histologic approach, the number of transverse sections of the penis with internalization of the urethra was related to the total length of the penis, achieving a percentage of urethral internalization. In this study, ≤89% of urethral internalization was defined as indicative of mild hypospadias (Stewart et al., 2018).
Regulatory Significance of the AO
In the OECD guidelines for developmental and reproductive toxicology, several test endpoints include examination of structural abnormalities with special attention to the organs of the reproductive system. These are: Test No. 414 ‘Prenatal Developmental Toxicity Study’ (OECD, 2018a); Test No. 416 ‘Two-Generation Reproduction Toxicity’ (OECD, 2001) and Tests No. 421/422 ‘Reproduction/Developmental Toxicity Screening Test’ (OECD, 2016a, 2016b). In Test No. 443 ‘Extended One-Generation Reproductive Toxicity Study’ (OECD, 2018b), hypospadias is specifically mentioned as a genital abnormality to note.
References
Baskin, L. S. (2000). Hypospadias and urethral development. The Journal of Urology, 163(3), 951–956.
Baskin, L. S., & Ebbers, M. B. (2006). Hypospadias: Anatomy, etiology, and technique. Journal of Pediatric Surgery, 41(3), 463–472. https://doi.org/10.1016/j.jpedsurg.2005.11.059
Gredler, M. L., Larkins, C. E., Leal, F., Lewis, A. K., Herrera, A. M., Perriton, C. L., Sanger, T. J., & Cohn, M. J. (2014). Evolution of External Genitalia: Insights from Reptilian Development. Sexual Development, 8(5), 311–326. https://doi.org/10.1159/000365771
Mahawong, P., Sinclair, A., Li, Y., Schlomer, B., Rodriguez, E., Ferretti, M. M., Liu, B., Baskin, L. S., & Cunha, G. R. (2014). Prenatal diethylstilbestrol induces malformation of the external genitalia of male and female mice and persistent second-generation developmental abnormalities of the external genitalia in two mouse strains. Differentiation, 88(2–3), 51–69. https://doi.org/10.1016/j.diff.2014.09.005
Mattiske, D. M., & Pask, A. J. (2021). Endocrine disrupting chemicals in the pathogenesis of hypospadias; developmental and toxicological perspectives. Current Research in Toxicology, 2, 179–191. https://doi.org/10.1016/j.crtox.2021.03.004
OECD. (2001). Test No. 416: Two-Generation Reproduction Toxicity. In OECD Guidelines for the Testing of Chemicals, Section 4. OECD Publishing. https://doi.org/10.1787/9789264070868-en
OECD. (2018). Test No. 414: Prenatal Developmental Toxicity Study. In OECD Guidelines for the Testing of Chemicals, Section 4. OECD Publishing. https://doi.org/10.1787/9789264070820-en
OECD. (2025a). Test No. 421: Reproduction/Developmental Toxicity Screening Test. In OECD Guidelines for the Testing of Chemicals, Section 4. OECD Publishing. https://doi.org/doi.org/10.1787/9789264264380-en
OECD. (2025b). Test No. 422: Combined Repeated Dose Toxicity Study with the Reproduction/Developmental Toxicity Screening Test. In OECD Guidelines for the Testing of Chemicals, Section 4. OECD Publising. https://doi.org/doi.org/10.1787/9789264264403-en
OECD. (2025c). Test No. 443: Extended One-Generation Reproductive Toxicity Study. In OECD Guidelines for the Testing of Chemicals, Section 4. OECD Publishing. https://doi.org/doi.org/10.1787/9789264185371-en
Paulozzi, L. J. (1999). International Trends in Rates of Hypospadias and Cryptorchidism. Environmental Health Perspectives, 107(4), 297–302. https://doi.org/10.1289/ehp.99107297
Sinclair, A. W., Cao, M., Pask, A., Baskin, L., & Cunha, G. R. (2017). Flutamide-induced hypospadias in rats: A critical assessment. Differentiation, 94, 37–57. https://doi.org/10.1016/j.diff.2016.12.001
Skakkebaek, N. E., Rajpert-De Meyts, E., Buck Louis, G. M., Toppari, J., Andersson, A.-M., Eisenberg, M. L., Jensen, T. K., Jørgensen, N., Swan, S. H., Sapra, K. J., Ziebe, S., Priskorn, L., & Juul, A. (2016). Male Reproductive Disorders and Fertility Trends: Influences of Environment and Genetic Susceptibility. Physiological Reviews, 96(1), 55–97. https://doi.org/10.1152/physrev.00017.2015.-It
Springer, A., van den Heijkant, M., & Baumann, S. (2016). Worldwide prevalence of hypospadias. Journal of Pediatric Urology, 12(3), 152.e1-152.e7. https://doi.org/10.1016/j.jpurol.2015.12.002
Stewart, M. K., Mattiske, D. M., & Pask, A. J. (2018). In utero exposure to both high- and low-dose diethylstilbestrol disrupts mouse genital tubercle development. Biology of Reproduction, 99(6), 1184–1193. https://doi.org/10.1093/biolre/ioy142
Uda, A., Kojima, Y., Hayashi, Y., Mizuno, K., Asai, N., & Kohri, K. (2004). Morphological features of external genitalia in hypospadiac rat model: 3-dimensional analysis. The Journal of Urology, 171(3), 1362–1366. https://doi.org/10.1097/01.JU.0000100140.42618.54
Vilela, M. L. B., Willingham, E., Buckley, J., Liu, B. C., Agras, K., Shiroyanagi, Y., & Baskin, L. S. (2007). Endocrine Disruptors and Hypospadias: Role of Genistein and the Fungicide Vinclozolin. Urology, 70(3), 618–621. https://doi.org/10.1016/j.urology.2007.05.004
Yu, X., Nassar, N., Mastroiacovo, P., Canfield, M., Groisman, B., Bermejo-Sánchez, E., Ritvanen, A., Kiuru-Kuhlefelt, S., Benavides, A., Sipek, A., Pierini, A., Bianchi, F., Källén, K., Gatt, M., Morgan, M., Tucker, D., Canessa, M. A., Gajardo, R., Mutchinick, O. M., … Agopian, A. J. (2019). Hypospadias Prevalence and Trends in International Birth Defect Surveillance Systems, 1980–2010. European Urology, 76(4), 482–490. https://doi.org/10.1016/j.eururo.2019.06.027
Appendix 2
List of Key Event Relationships in the AOP
List of Adjacent Key Event Relationships
Relationship: 3448: Decrease, intratesticular testosterone leads to Decrease, circulating testosterone levels
AOPs Referencing Relationship
| AOP Name | Adjacency | Weight of Evidence | Quantitative Understanding |
|---|---|---|---|
| Decreased testosterone synthesis leading to short anogenital distance (AGD) in male (mammalian) offspring | adjacent | High | Moderate |
| Decreased testosterone synthesis leading to hypospadias in male (mammalian) offspring | adjacent | High | |
| Decreased testosterone synthesis leading to increased nipple retention (NR) in male (rodent) offspring | adjacent | High |
Evidence Supporting Applicability of this Relationship
| Life Stage | Evidence |
|---|---|
| All life stages | High |
| Sex | Evidence |
|---|---|
| Male | High |
Taxonomic applicability
The KER is assessed applicable to mammals, as testicular testosterone synthesis is common for all mammals. It is, however, acknowledged that this KER most likely has a much broader domain of applicability extending to non-mammalian vertebrates.
Sex applicability
This KER is only applicable to males, as testes are only found in males.
Life stage applicability
This KER is applicable to all life stages. Once formed, the testes produce and secrete testosterone during fetal development and throughout postnatal life, although testosterone levels do vary between life stages (Vesper et al., 2015).
Key Event Relationship Description
This KE describes a decrease in intratesticular testosterone production leading to a decrease in circulating levels of testosterone. Intratesticular testosterone can be measured in whole testicular tissue samples by testing ex vivo testicular testosterone production, and circulating testosterone is measured in plasma or serum. In males, the testes produce and secrete the majority of the circulating testosterone, with only a small contribution from the adrenal gland (Naamneh Elzenaty et al., 2022). In mammals, intratesticular testosterone levels are 30- to 100-fold higher than serum testosterone levels (Coviello et al., 2004; McLachlan et al., 2002; Turner et al., 1984). Reducing testicular testosterone will consequently lead to a reduction in circulating levels as well.
Evidence Supporting this KER
Biological PlausibilityThe biological plausibility for this KER is considered high. The testes are the primary testosterone-producing organs in male mammals and the main contributors to the circulating testosterone levels in males (Naamneh Elzenaty et al., 2022). A decrease in intratesticular testosterone will therefore lead to a decrease in secretion of testosterone and consequently lower circulating levels of testosterone.
Empirical EvidenceThe empirical evidence for this KER is overall judged as high.
In vivo toxicity studies in rats and mice have shown that exposure to substances that lowers intratesticular testosterone also lowers circulating testosterone levels. This includes in utero exposure and measurements in fetal males (Borch J et al., 2004; Vinggaard AM et al., 2005) as well as exposure and measurements postnatally in male rodents (Hou X et al., 2020; Ji et al., 2010; Jiang XP et al., 2017)
Supporting this evidence are castration studies in male rats and monkeys, showing a marked reduction in circulating testosterone levels when removing the testes (Gomes & Jain, 1976; Perachio et al., 1977).
Lastly, in humans, males with hypogonadism or gonadal dysgenesis present with lower circulating testosterone levels (Hirose Y et al., 2007; Jones LW et al., 1970).
Dose concordance
In vivo toxicity studies support dose concordance for this KER, as exemplified below.
In pre-pubertal/pubertal male rats, chlorocholine chloride exposure (postnatal day (PND) 23-60) in three doses reduced both intratesticular and serum testosterone levels at PND60 at all doses tested (Hou X et al., 2020).
Perinatal exposure (gestational day (GD) 10-birth) of male mice to diethylhexyl phthalate (DEHP) in three doses (100, 500, and 1000 mg/kg bw/day) reduced intratesticular testosterone at 500 and 1000 mg/kg bw/day at PND1, while only 1000 mg/kg bw/day reduced serum levels of testosterone, although this was measured later, at PND56 (Xie Q et al., 2024)
In utero exposure (GD7-21) of male rats to DEHP in doses of 300 or 750 mg/kg bw/day reduced intratesticular testosterone levels at GD21, while only the high dose also reduced plasma testosterone levels (Borch J et al., 2004).
Temporal concordance
In vivo toxicity studies moderately support temporal concordance for this KER, as exemplified below.
Several studies show that a decrease in intratesticular and circulating testosterone can be measured at the same time point (Borch J et al., 2004; Hou X et al., 2020; Jiang XP et al., 2017; Vinggaard AM et al., 2005).
In utero exposure of male mice to DEHP from GD10 to birth reduced intratesticular testosterone levels at PND1 with LOAEL 500 mg/kg bw/day, and when measured at PND56, circulating testosterone levels were decreased, but with LOAEL 1000 mg/kg bw/day (Xie Q et al., 2024).
In Fisher JS et al., 2003, exposure of male rats from GD13-21 to 500 mg/kg bw/day dibutyl phthalate reduced intratesticular testosterone by ~90% (measured at GD19). When analyzing circulating testosterone levels at PND4, 10, 15, 25, and 90, only the testosterone levels on PND25 were decreased.
One study report conflicting results on the temporal concordance of this KER (Caceres et al., 2023). Here, male rats were exposed for 20 weeks from PND60 to a mixture of the phytoestrogens genistein and daidzein (combined dose of either 29 or 290 mg/kg bw/day). Intratesticular testosterone was measured every 4 weeks, while serum levels of testosterone were measured every second week. While the mixture caused a reduction of serum testosterone after 2 weeks of exposure, a reduction in intratesticular testosterone was not measured until after 8 weeks. The discrepancy might be explained by the multiple mechanisms of action of the phytoestrogens, as they, besides affecting testicular testosterone synthesis, may also influence peripheral aromatization of testosterone to estrogens (van Duursen et al., 2011).
Incidence concordance
Incidence concordance can not be evaluated for this KER.
Uncertainties and InconsistenciesThere are examples of in vivo studies, in which stressors exposure have caused a reduction in intratesticular testosterone levels without a reduction in circulating testosterone levels.
Quantitative Understanding of the Linkage
Time-scaleThe time-scale for this KER is likely minutes or hours, as testosterone is secreted into the blood from the testes after synthesis. In vivo, a decrease in intratesticular and circulating testosterone can be measured at the same time, both in fetal and postnatal studies (Borch J et al., 2004; Hou X et al., 2020; Jiang XP et al., 2017; Vinggaard AM et al., 2005). Ex vivo, chemically-induced reduction in testicular production of testosterone can be measured in culture media after 3 hours incubation (earlier time points were not measured) (Wilson et al., 2009).
Known Feedforward/Feedback loops influencing this KERTestosterone is a part of the hypothalamic-pituitary-gonadal (HPG) axis, which controls testosterone synthesis in puberty and adulthood. In this axis, gonatropin-releasing hormone (GnRH) is released from the hypothalamus and stimulates release of luteinizing hormone (LH) from the pituitary. LH acts on the testes to produce and secrete testosterone. Elevated circulating testosterone levels exerts negative feedback on the HPG axis (decreasing GnRH secretion) to keep testosterone levels in balance (Tilbrook & Clarke, 2001).
Importantly, there are species-specific differences in when the HPG axis is functional during development. In the mouse, fetal testosterone synthesis is independent of pituitary LH (O’Shaughnessy et al., 1998), whereas in humans, human chorionic gonadotropin (hCG) act similarly to LH and appear to be critical in stimulating testosterone synthesis in the fetal testis (Huhtaniemi, 2025).
References
Borch J, Ladefoged O, Hass U, & Vinggaard AM. (2004). Steroidogenesis in fetal male rats is reduced by DEHP and DINP, but endocrine effects of DEHP are not modulated by DEHA in fetal, prepubertal and adult male rats. Reproductive Toxicology (Elmsford, N.Y.), 18(1), 53–61. https://doi.org/10.1016/j.reprotox.2003.10.011
Caceres, S., Crespo, B., Alonso-Diez, A., De Andrés, P. J., Millan, P., Silván, G., Illera, M. J., & Illera, J. C. (2023). Long-Term Exposure to Isoflavones Alters the Hormonal Steroid Homeostasis-Impairing Reproductive Function in Adult Male Wistar Rats. Nutrients, 15(5), 1261. https://doi.org/10.3390/nu15051261
Coviello, A. D., Bremner, W. J., Matsumoto, A. M., Herbst, K. L., Amory, J. K., Anawalt, B. D., Yan, X., Brown, T. R., Wright, W. W., Zirkin, B. R., & Jarow, J. P. (2004). Intratesticular Testosterone Concentrations Comparable With Serum Levels Are Not Sufficient to Maintain Normal Sperm Production in Men Receiving a Hormonal Contraceptive Regimen. Journal of Andrology, 25(6), 931–938. https://doi.org/10.1002/j.1939-4640.2004.tb03164.x
Fisher JS, Macpherson S, Marchetti N, & Sharpe RM. (2003). Human “testicular dysgenesis syndrome”: A possible model using in-utero exposure of the rat to dibutyl phthalate. Human Reproduction (Oxford, England), 18(7), 1383–1394. https://doi.org/10.1093/humrep/deg273
Gomes, W. R., & Jain, S. K. (1976). Effect of unilateral and bilateral castration and cryptorchidism on serum gonadotrophins in the rat. The Journal of Endocrinology, 68(02), 191–196. https://doi.org/10.1677/joe.0.0680191
Hirose Y, Sasa M, Bando Y, Hirose T, Morimoto T, Kurokawa Y, Nagao T, & Tangoku A. (2007). Bilateral male breast cancer with male potential hypogonadism. World Journal of Surgical Oncology, 5, 60. https://doi.org/10.1186/1477-7819-5-60
Hou X, Hu H, Xiagedeer B, Wang P, Kang C, Zhang Q, Meng Q, & Hao W. (2020). Effects of chlorocholine chloride on pubertal development and reproductive functions in male rats. Toxicology Letters, 319, 1–10. https://doi.org/10.1016/j.toxlet.2019.10.024
Huhtaniemi, I. T. (2025). Luteinizing hormone receptor knockout mouse: What has it taught us? Andrology, andr.70000. https://doi.org/10.1111/andr.70000
Ji, Y.-L., Wang, H., Liu, P., Wang, Q., Zhao, X.-F., Meng, X.-H., Yu, T., Zhang, H., Zhang, C., Zhang, Y., & Xu, D.-X. (2010). Pubertal cadmium exposure impairs testicular development and spermatogenesis via disrupting testicular testosterone synthesis in adult mice. Reproductive Toxicology, 29(2), 176–183. https://doi.org/10.1016/j.reprotox.2009.10.014
Jiang XP, Tang JY, Xu Z, Han P, Qin ZQ, Yang CD, Wang SQ, Tang M, Wang W, Qin C, Xu Y, Shen BX, Zhou WM, & Zhang W. (2017). Sulforaphane attenuates di-N-butylphthalate-induced reproductive damage in pubertal mice: Involvement of the Nrf2-antioxidant system. Environmental Toxicology, 32(7), 1908–1917. https://doi.org/10.1002/tox.22413
Jones LW, Isaacs H Jr, Edelbrock H, & Donnell GN. (1970). Reifenstein’s syndrome: Hereditary familial hypogonadism with hypospadias and gynecomastia. The Journal of Urology, 104(4), 608–611. https://doi.org/10.1016/s0022-5347(17)61793-2
McLachlan, R. I., O’Donnell, L., Stanton, P. G., Balourdos, G., Frydenberg, M., de Kretser, D. M., & Robertson, D. M. (2002). Effects of Testosterone Plus Medroxyprogesterone Acetate on Semen Quality, Reproductive Hormones, and Germ Cell Populations in Normal Young Men. The Journal of Clinical Endocrinology & Metabolism, 87(2), 546–556. https://doi.org/10.1210/jcem.87.2.8231
Naamneh Elzenaty, R., Du Toit, T., & Flück, C. E. (2022). Basics of androgen synthesis and action. Best Practice & Research Clinical Endocrinology & Metabolism, 36(4), 101665. https://doi.org/10.1016/j.beem.2022.101665
O’Shaughnessy, P. J., Baker, P., Sohnius, U., Haavisto, A.-M., Charlton, H. M., & Huhtaniemi, I. (1998). Fetal Development of Leydig Cell Activity in the Mouse Is Independent of Pituitary Gonadotroph Function*. Endocrinology, 139(3), 1141–1146. https://doi.org/10.1210/endo.139.3.5788
Perachio, A. A., Alexander, M., Marr, L. D., & Collins, D. C. (1977). Diurnal variations of serum testosterone levels in intact and gonadectomized male and female rhesus monkeys. Steroids, 29(1), 21–33. https://doi.org/10.1016/0039-128X(77)90106-4
Tilbrook, A. J., & Clarke, I. J. (2001). Negative Feedback Regulation of the Secretion and Actions of Gonadotropin-Releasing Hormone in Males. Biology of Reproduction, 64(3), 735–742. https://doi.org/10.1095/biolreprod64.3.735
Turner, T. T., Jones, C. E., Howards, S. S., Ewing, L. L., Zegeye, B., & Gunsalus, G. L. (1984). On the androgen microenvironment of maturing spermatozoa. Endocrinology, 115(5), 1925–1932. https://doi.org/10.1210/endo-115-5-1925
van Duursen, M. B. M., Nijmeijer, S. M., de Morree, E. S., de Jong, P. Chr., & van den Berg, M. (2011). Genistein induces breast cancer-associated aromatase and stimulates estrogen-dependent tumor cell growth in in vitro breast cancer model. Toxicology, 289(2), 67–73. https://doi.org/10.1016/j.tox.2011.07.005
Vesper, H. W., Wang, Y., Vidal, M., Botelho, J. C., & Caudill, S. P. (2015). Serum Total Testosterone Concentrations in the US Household Population from the NHANES 2011-2012 Study Population. Clinical Chemisty, 61(12), 1495–1504. https://doi.org/10.1373/clinchem.2015.245969
Vinggaard AM, Christiansen S, Laier P, Poulsen ME, Breinholt V, Jarfelt K, Jacobsen H, Dalgaard M, Nellemann C, & Hass U. (2005). Perinatal exposure to the fungicide prochloraz feminizes the male rat offspring. Toxicological Sciences : An Official Journal of the Society of Toxicology, 85(2), 886–897. https://doi.org/doi.org/10.1093/toxsci/kfi150
Wilson, V. S., Lambright, C. R., Furr, J. R., Howdeshell, K. L., & Gray, L. E., Jr. (2009). The herbicide linuron reduces testosterone production from the fetal rat testis during both in utero and in vitro exposures. TOXICOLOGY LETTERS, 186(2), 73–77. https://doi.org/10.1016/j.toxlet.2008.12.017
Xie Q, Cao H, Liu H, Xia K, Gao Y, & Deng C. (2024). Prenatal DEHP exposure induces lifelong testicular toxicity by continuously interfering with steroidogenic gene expression. Translational Andrology and Urology, 13(3), 369–382. https://doi.org/10.21037/tau-23-503
Relationship: 2131: Decrease, circulating testosterone levels leads to Decrease, AR activation
AOPs Referencing Relationship
| AOP Name | Adjacency | Weight of Evidence | Quantitative Understanding |
|---|---|---|---|
| Inhibition of 17α-hydrolase/C 10,20-lyase (Cyp17A1) activity leads to birth reproductive defects (cryptorchidism) in male (mammals) | adjacent | High | High |
| Decreased testosterone synthesis leading to short anogenital distance (AGD) in male (mammalian) offspring | adjacent | High | Moderate |
| Decreased testosterone synthesis leading to hypospadias in male (mammalian) offspring | adjacent | High | |
| Decreased testosterone synthesis leading to increased nipple retention (NR) in male (rodent) offspring | adjacent | High |
Evidence Supporting Applicability of this Relationship
| Term | Scientific Term | Evidence | Links |
|---|---|---|---|
| mammals | mammals | High | NCBI |
| Life Stage | Evidence |
|---|---|
| During development and at adulthood | High |
| Sex | Evidence |
|---|---|
| Mixed | High |
Taxonomic applicability
KER2131 is assessed applicable to mammals, as T and AR activation are known to be related in mammals. It is, however, acknowledged that this KER most likely has a much broader domain of applicability extending to non-mammalian vertebrates. AOP developers are encouraged to add additional relevant knowledge to expand on the applicability to also include other vertebrates.
Sex applicability
KER2131 is assessed applicable to both sexes, as T activates AR in both males and females.
Life-stage applicability
KER2131 is considered applicable to developmental and adult life stages, as T-mediated AR activation is relevant from the AR is expressed.
Key Event Relationship Description
This key event relationship links decreased testosterone (T) levels to decreased androgen receptor (AR) activation. T is an endogenous steroid hormone important for, amongst other things, reproductive organ development and growth as well as muscle mass and spermatogenesis (Marks, 2004).T is, together with dihydrotestosterone (DHT), a primary ligand for the AR in mammals (Schuppe et al., 2020). Besides its genomic actions, the AR can also mediate rapid, non-genomic second messenger signaling (Davey & Grossmann, 2016). When T levels are reduced, less substrate is available for the AR, and hence, AR activation is decreased (Gao et al., 2005).
Evidence Supporting this KER
Biological PlausibilityThe biological plausibility for this KER is considered high
AR activation is dependent on ligand binding (though a few cases of ligand-independent AR activation has been shown, see uncertainties and inconsistencies). T is a primary ligand for the AR, and when T levels are decreased there is less substrate for the AR, and hence, AR activation is decreased. In the male, T is primarily synthesized by the testes, and in some target tissues T is irreversibly metabolized to the more potent metabolite DHT. T and DHT both bind to the AR, but DHT has a higher binding affinity (Gao et al., 2005). The lower binding affinity of T compared to DHT is due to the faster dissociation rate of T from the full-length AR, as T has less effective FXXLF motif binding to AF2 (Askew et al., 2007). Binding of T or DHT has different effects in different tissues. E.g. in the developing male, T is required for development of the internal sex organs (epididymis, vas deferens and the seminal vesicles), whereas DHT is crucial for development of the external sex organs (Keller et al., 1996). In the adult male, androgen action in the reproductive tissues is DHT dependent, whereas action in muscle and bone is DHT independent (Gao et al., 2005). In patients with male androgen deficiency syndrome (AIS), clinically low levels of T leads to reduced AR activation (either due to low T or DHT in target tissue), which manifests as both androgenic related symptoms (such as incomplete or delayed sexual development, loss of body hair, small or shrinking testes, low or zero sperm count) as well as anabolic related symptoms (such as height loss, low trauma fracture, low bone mineral density, reduced muscle bulk and strength, increased body fat). All symptoms can be counteracted by treatment with T, which acts directly on the AR receptor in anabolic tissue (Bhasin et al., 2010). Similarly, removal of the testicles in weanling rats results in a feminized body composition and muscle metabolism, which is reversed by administration of T (Krotkiewski et al., 1980). As this demonstrates, the consequences of low T regarding AR activation will depend on tissue, life stage, species etc.
Empirical EvidenceThe empirical evidence for this KER is considered high
Dose concordance
There is a positive dose-response relationship between increasing concentrations of T and AR activation (U.S. EPA., 2023).
Other evidence
- In male patients with androgen deficiency, treatment with T counteracts anabolic (DHT independent) related symptoms such as height loss, low trauma fracture, low bone mineral density, reduced muscle bulk and strength, increased body fat (Bhasin et al., 2010; Katznelson et al., 1996).
- Removal of the testicles in weanling rats result in a feminized body composition and muscle metabolism, which is reversed by administration of T (Krotkiewski et al., 1980).
Ligand-independent actions of the AR have been identified. To what extent and of which biological significance is not well defined (Bennesch & Picard, 2015).
Quantitative Understanding of the Linkage
Response-response relationshipThere is a positive dose-response relationship between increasing concentrations of T and AR activation (U.S. EPA., 2023). However, there is not enough data, or overview of the data, to define a quantitative linkage in vivo, and such a relationship will differ between biological systems (species, tissue, cell type).
Time-scaleAR and promoter interactions occur within 15 minutes of ligand binding, and RNA polymerase II and coactivator recruitment are then proposed to occur transiently with cycles of approximately 90 minutes (Kang et al., 2002).
Known modulating factors| Modulating Factor (MF) | MF Specification | Effect(s) on the KER | Reference(s) |
|---|---|---|---|
| Age | AR expression changes with aging | Tissue-specific alterations in AR activity with aging | (Supakar et al., 1993; Wu et al., 2009) |
| Genotype | Number of CAG repeats in the first exon of AR | Decreased AR activation with increased number of CAGs | (Chamberlain et al., 1994; Tut et al., 1997) |
| Male androgen deficiency syndrome | Low circulating testosterone levels due to primary (testicular) or secondary (pituitary-hypothalamic) hypogonadism | Reduced levels of circulating testosterone | (Bhasin et al., 2010) |
| Castration | Removal of testicles | Reduced levels of circulating testosterone | (Krotkiewski et al., 1980) |
Androgens can upregulate and downregulate AR expression (Lee & Chang, 2003).
References
Askew, E. B., Gampe, R. T., Stanley, T. B., Faggart, J. L., & Wilson, E. M. (2007). Modulation of Androgen Receptor Activation Function 2 by Testosterone and Dihydrotestosterone. Journal of Biological Chemistry, 282(35), 25801–25816. https://doi.org/10.1074/jbc.M703268200
Bennesch, M. A., & Picard, D. (2015). Minireview: Tipping the Balance: Ligand-Independent Activation of Steroid Receptors. Molecular Endocrinology, 29(3), 349–363. https://doi.org/10.1210/me.2014-1315
Bhasin, S., Cunningham, G. R., Hayes, F. J., Matsumoto, A. M., Snyder, P. J., Swerdloff, R. S., & Montori, V. M. (2010). Testosterone Therapy in Men with Androgen Deficiency Syndromes: An Endocrine Society Clinical Practice Guideline. The Journal of Clinical Endocrinology & Metabolism, 95(6), 2536–2559. https://doi.org/10.1210/jc.2009-2354
Davey, R. A., & Grossmann, M. (2016). Androgen Receptor Structure, Function and Biology: From Bench to Bedside. The Clinical Biochemist. Reviews, 37(1), 3–15. http://www.ncbi.nlm.nih.gov/pubmed/27057074
Gao, W., Bohl, C. E., & Dalton, J. T. (2005). Chemistry and Structural Biology of Androgen Receptor. Chemical Reviews, 105(9), 3352–3370. https://doi.org/10.1021/cr020456u
Kang, Z., Pirskanen, A., Jänne, O. A., & Palvimo, J. J. (2002). Involvement of Proteasome in the Dynamic Assembly of the Androgen Receptor Transcription Complex. Journal of Biological Chemistry, 277(50), 48366–48371. https://doi.org/10.1074/jbc.M209074200
Katznelson, L., Finkelstein, J. S., Schoenfeld, D. A., Rosenthal, D. I., Anderson, E. J., & Klibanski, A. (1996). Increase in bone density and lean body mass during testosterone administration in men with acquired hypogonadism. The Journal of Clinical Endocrinology & Metabolism, 81(12), 4358–4365. https://doi.org/10.1210/jcem.81.12.8954042
Keller, E. T., Ershler, W. B., & Chang, Chawnshang. (1996). The androgen receptor: A mediator of diverse responses. Frontiers in Bioscience, 1(4), 59–71. https://doi.org/10.2741/A116
Krotkiewski, M., Kral, J. G., & Karlsson, J. (1980). Effects of castration and testosterone substitution on body composition and muscle metabolism in rats. Acta Physiologica Scandinavica, 109(3), 233–237. https://doi.org/10.1111/j.1748-1716.1980.tb06592.x
Lee, D. K., & Chang, C. (2003). Expression and Degradation of Androgen Receptor: Mechanism and Clinical Implication. The Journal of Clinical Endocrinology & Metabolism, 88(9), 4043–4054. https://doi.org/10.1210/jc.2003-030261
Marks, L. S. (2004). 5alpha-reductase: history and clinical importance. Reviews in Urology, 6 Suppl 9(Suppl 9), S11-21. http://www.ncbi.nlm.nih.gov/pubmed/16985920
Schuppe, E. R., Miles, M. C., and Fuxjager, M. J. (2020). Evolution of the androgen receptor: Perspectives from human health to dancing birds. Mol. Cell. Endocrinol. 499, 110577. doi:10.1016/J.MCE.2019.110577.
U.S. EPA. (2023). ToxCast & Tox21 AR agonism of testosterone. Retrieved from Https://Www.Epa.Gov/Chemical-Research/Toxicity-Forecaster-Toxcasttm-Data June 23, 2023. Data Released October 2018.
Relationship: 2124: Decrease, AR activation leads to Altered, Transcription of genes by the AR
AOPs Referencing Relationship
Evidence Supporting Applicability of this Relationship
| Term | Scientific Term | Evidence | Links |
|---|---|---|---|
| mammals | mammals | High | NCBI |
| Life Stage | Evidence |
|---|---|
| During development and at adulthood | High |
| Sex | Evidence |
|---|---|
| Mixed | High |
This KER is applicable for both sexes, across developmental stages into adulthood, in numerous cells and tissues and across mammalian taxa. It is, however, acknowledged that this KER most likely has a much broader domain of applicability extending to non-mammalian vertebrates. AOP developers are encouraged to add additional relevant knowledge to expand on the applicability to also include other vertebrates.
Key Event Relationship Description
The androgen receptor (AR) is a ligand-dependent nuclear transcription factor that upon activation translocates to the nucleus, dimerizes, and binds androgen response elements (AREs) to modulate transcription of target genes (Lamont and Tindall, 2010, Roy et al. 2001). Decreased activation of the AR affects its transcription factor activity, therefore leading to altered AR-target gene expression. This KER refers to decreased AR activation and altered gene expression occurring in complex systems, such as in vivo and the specific effect on transcription of AR target genes will depend on species, life stage, tissue, cell type etc.
Evidence Supporting this KER
Biological PlausibilityThe biological plausibility for this KER is considered high
The AR is a ligand-activated transcription factor part of the steroid hormone nuclear receptor family. Non-activated AR is found in the cytoplasm as a multiprotein complex with heat-shock proteins, immunophilins and, other chaperones (Roy et al. 2001). Upon activation through ligand binding, the AR dissociates from the protein complex, translocates to the nucleus and homodimerizes. Facilitated by co-regulators, AR can bind to DNA regions containing AREs and initiate transcription of target genes, that thus will be different in e.g. different tissues, life-stages, species etc.
Through mapping of AREs and ChIP sequencing studies, several AR target genes have been identified, mainly studied in prostate cells (Jin, Kim, and Yu 2013). Different co-regulators and ligands lead to altered expression of different sets of genes (Jin et al. 2013; Kanno et al. 2022). Alternative splicing of the AR can lead to different AR variants that also affects which genes are transcribed (Jin et al. 2013).
Apart from this canonical signaling pathway, the AR can suppress gene expression, indirectly regulate miRNA transcription, and have non-genomic effects by rapid activation of second messenger pathways in either presence or absence of a ligand (Jin et al. 2013).
Empirical EvidenceThe empirical evidence for this KER is considered high
In humans, altered gene expression profiling in individuals with androgen insensitivity syndrome (AIS) can provide supporting empirical evidence (Holterhus et al. 2003; Peng et al. 2021). In rodent AR knockout (KO) models, gene expression profiling studies and gene-targeted approaches have provided information on differentially expressed genes in several organ systems including male and female reproductive, endocrine, muscular, cardiovascular and nervous systems (Denolet et al. 2006; Fan et al. 2005; Holterhus et al. 2003; Ikeda et al. 2005; Karlsson et al. 2016; MacLean et al. 2008; Rana et al. 2011; Russell et al. 2012; Shiina et al. 2006; Wang et al. 2006; Welsh et al. 2012; Willems et al. 2010; Yu et al. 2008, 2012; Zhang et al. 2006; Zhou et al. 2011).
Exposure to known antiandrogens has been shown to alter transcriptional profiles, for example of neonatal pig ovaries (Knapczyk-Stwora et al. 2019).
Dose concordance has also been observed for instance in zebrafish embryos; a dose of 50 µg/L of the AR antagonist flutamide resulted in 674 differentially expressed genes at 96 h post fertilization whereas 500 µg/L flutamide resulted in 2871 differentially expressed genes (Ayobahan et al., 2023).
Uncertainties and InconsistenciesAR action has been reported to occur also without ligand binding. However, not much is known about the extent and biological implications of such non-canonical, ligand-independent AR activation (Bennesch and Picard 2015).
Quantitative Understanding of the Linkage
Response-response relationshipThere is not enough data to define a quantitative relationship between AR activation and alteration of AR target gene transcription, and such a relationship will differ between biological systems (species, tissue, cell type, life stage etc).
Time-scaleAR and promoter interactions occur within 15 minutes of ligand binding, RNA polymerase II and coactivator recruitment are proposed to occur transiently with cycles of approximately 90 minutes in LNCaP cells (Kang et al. 2002). RNA polymerase II elongation rates in mammalian cells have been shown to range between 1.3 and 4.3 kb/min (Maiuri et al. 2011). Therefore, depending on the cell type and the half-life of the AR target gene transcripts, changes are to be expected within hours.
Known modulating factors| Modulating Factor (MF) | MF Specification | Effect(s) on the KER | Reference(s) |
|---|---|---|---|
| Age | AR expression in aging male rats | Tissue-specific alterations in AR activity with aging | (Supakar et al. 1993; Wu, Lin, and Gore 2009) |
| Genotype | Number of CAG repeats in the first exon of AR | Decreased AR activation with increased number of CAGs |
(Tut et al. 1997; Chamberlain et al. 1994) |
AR has been hypothesized to auto-regulate its mRNA and protein levels (Mora and Mahesh 1999).
References
Ayobahan, S. U., Alvincz, J., Reinwald, H., Strompen, J., Salinas, G., Schäfers, C., et al. (2023). Comprehensive identification of gene expression fingerprints and biomarkers of sexual endocrine disruption in zebrafish embryo. Ecotoxicol. Environ. Saf. 250, 114514. doi:10.1016/J.ECOENV.2023.114514.
Bennesch, Marcela A., and Didier Picard. 2015. “Minireview: Tipping the Balance: Ligand-Independent Activation of Steroid Receptors.” Molecular Endocrinology 29(3):349–63.
Chamberlain, Nancy L., Erika D. Driverand, and Roger L. Miesfeldi. 1994. The Length and Location of CAG Trinucleotide Repeats in the Androgen Receptor N-Terminal Domain Affect Transactivation Function. Vol. 22.
Denolet, Evi, Karel De Gendt, Joke Allemeersch, Kristof Engelen, Kathleen Marchal, Paul Van Hummelen, Karen A. L. Tan, Richard M. Sharpe, Philippa T. K. Saunders, Johannes V. Swinnen, and Guido Verhoeven. 2006. “The Effect of a Sertoli Cell-Selective Knockout of the Androgen Receptor on Testicular Gene Expression in Prepubertal Mice.” Molecular Endocrinology 20(2):321–34. doi: 10.1210/me.2005-0113.
Fan, Wuqiang, Toshihiko Yanase, Masatoshi Nomura, Taijiro Okabe, Kiminobu Goto, Takashi Sato, Hirotaka Kawano, Shigeaki Kato, and Hajime Nawata. 2005. Androgen Receptor Null Male Mice Develop Late-Onset Obesity Caused by Decreased Energy Expenditure and Lipolytic Activity but Show Normal Insulin Sensitivity With High Adiponectin Secretion. Vol. 54.
Holterhus, Paul-Martin, Olaf Hiort, Janos Demeter, Patrick O. Brown, and James D. Brooks. 2003. Differential Gene-Expression Patterns in Genital Fibroblasts of Normal Males and 46,XY Females with Androgen Insensitivity Syndrome: Evidence for Early Programming Involving the Androgen Receptor. Vol. 4.
Ikeda, Yasumasa, Ken Ichi Aihara, Takashi Sato, Masashi Akaike, Masanori Yoshizumi, Yuki Suzaki, Yuki Izawa, Mitsunori Fujimura, Shunji Hashizume, Midori Kato, Shusuke Yagi, Toshiaki Tamaki, Hirotaka Kawano, Takahiro Matsumoto, Hiroyuki Azuma, Shigeaki Kato, and Toshio Matsumoto. 2005. “Androgen Receptor Gene Knockout Male Mice Exhibit Impaired Cardiac Growth and Exacerbation of Angiotensin II-Induced Cardiac Fibrosis.” Journal of Biological Chemistry 280(33):29661–66. doi: 10.1074/jbc.M411694200.
Jin, Hong Jian, Jung Kim, and Jindan Yu. 2013. “Androgen Receptor Genomic Regulation.” Translational Andrology and Urology 2(3):158–77.
Kang, Zhigang, Asta Pirskanen, Olli A. Jänne, and Jorma J. Palvimo. 2002. “Involvement of Proteasome in the Dynamic Assembly of the Androgen Receptor Transcription Complex.” Journal of Biological Chemistry 277(50):48366–71. doi: 10.1074/jbc.M209074200.
Kanno, Yuichiro, Nao Saito, Ryota Saito, Tomohiro Kosuge, Ryota Shizu, Tomofumi Yatsu, Takuomi Hosaka, Kiyomitsu Nemoto, Keisuke Kato, and Kouichi Yoshinari. 2022. “Differential DNA-Binding and Cofactor Recruitment Are Possible Determinants of the Synthetic Steroid YK11-Dependent Gene Expression by Androgen Receptor in Breast Cancer MDA-MB 453 Cells.” Experimental Cell Research 419(2). doi: 10.1016/j.yexcr.2022.113333.
Karlsson, Sara A., Erik Studer, Petronella Kettunen, and Lars Westberg. 2016. “Neural Androgen Receptors Modulate Gene Expression and Social Recognition but Not Social Investigation.” Frontiers in Behavioral Neuroscience 10(MAR). doi: 10.3389/fnbeh.2016.00041.
Knapczyk-Stwora, Katarzyna, Anna Nynca, Renata E. Ciereszko, Lukasz Paukszto, Jan P. Jastrzebski, Elzbieta Czaja, Patrycja Witek, Marek Koziorowski, and Maria Slomczynska. 2019. “Flutamide-Induced Alterations in Transcriptional Profiling of Neonatal Porcine Ovaries.” Journal of Animal Science and Biotechnology 10(1):1–15. doi: 10.1186/s40104-019-0340-y.
Lamont, K. R., and Tindall, D. J. (2010). Androgen Regulation of Gene Expression. Adv. Cancer Res. 107, 137–162. doi:10.1016/S0065-230X(10)07005-3.
MacLean, Helen E., W. S. Maria Chiu, Amanda J. Notini, Anna-Maree Axell, Rachel A. Davey, Julie F. McManus, Cathy Ma, David R. Plant, Gordon S. Lynch, and Jeffrey D. Zajac. 2008. “ Impaired Skeletal Muscle Development and Function in Male, but Not Female, Genomic Androgen Receptor Knockout Mice .” The FASEB Journal 22(8):2676–89. doi: 10.1096/fj.08-105726.
Maiuri, Paolo, Anna Knezevich, Alex De Marco, Davide Mazza, Anna Kula, Jim G. McNally, and Alessandro Marcello. 2011. “Fast Transcription Rates of RNA Polymerase II in Human Cells.” EMBO Reports 12(12):1280–85. doi: 10.1038/embor.2011.196.
Mora, Gloria R., and Virendra B. Mahesh. 1999. Autoregulation of the Androgen Receptor at the Translational Level: Testosterone Induces Accumulation of Androgen Receptor MRNA in the Rat Ventral Prostate Polyribosomes.
Peng, Yajie, Hui Zhu, Bing Han, Yue Xu, Xuemeng Liu, Huaidong Song, and Jie Qiao. 2021. “Identification of Potential Genes in Pathogenesis and Diagnostic Value Analysis of Partial Androgen Insensitivity Syndrome Using Bioinformatics Analysis.” Frontiers in Endocrinology 12. doi: 10.3389/fendo.2021.731107.
Rana, Kesha, Barbara C. Fam, Michele V Clarke, Tammy P. S. Pang, Jeffrey D. Zajac, and Helen E. Maclean. 2011. “Increased Adiposity in DNA Binding-Dependent Androgen Receptor Knockout Male Mice Associated with Decreased Voluntary Activity and Not Insulin Resistance.” Am J Physiol Endocrinol Me-Tab 301:767–78. doi: 10.1152/ajpendo.00584.2010.-In.
Roy, Arun K., Rakesh K. Tyagi, Chung S. Song, Yan Lavrovsky, Soon C. Ahn, Tae Sung Oh, and Bandana Chatterjee. 2001. “Androgen Receptor: Structural Domains and Functional Dynamics after Ligand-Receptor Interaction.” Pp. 44–57 in Annals of the New York Academy of Sciences. Vol. 949. New York Academy of Sciences.
Russell, Patricia K., Michele V. Clarke, Jarrod P. Skinner, Tammy P. S. Pang, Jeffrey D. Zajac, and Rachel A. Davey. 2012. “Identification of Gene Pathways Altered by Deletion of the Androgen Receptor Specifically in Mineralizing Osteoblasts and Osteocytes in Mice.” Journal of Molecular Endocrinology 49(1):1–10. doi: 10.1530/JME-12-0014.
Shiina, Hiroko, Takahiro Matsumoto, Takashi Sato, Katsuhide Igarashi, Junko Miyamoto, Sayuri Takemasa, Matomo Sakari, Ichiro Takada, Takashi Nakamura, Daniel Metzger, Pierre Chambon, Jun Kanno, Hiroyuki Yoshikawa, and Shigeaki Kato. 2006. Premature Ovarian Failure in Androgen Receptor-Deficient Mice. Vol. 103.
Supakar, P. C., C. S. Song, M. H. Jung, M. A. Slomczynska, J. M. Kim, R. L. Vellanoweth, B. Chatterjee, and A. K. Roy. 1993. “A Novel Regulatory Element Associated with Age-Dependent Expression of the Rat Androgen Receptor Gene.” Journal of Biological Chemistry 268(35):26400–408. doi: 10.1016/s0021-9258(19)74328-2.
Tut, Thein G., Farid J. Ghadessy, M. A. Trifiro, L. Pinsky, and E. L. Yong. 1997. Long Polyglutamine Tracts in the Androgen Receptor Are Associated with Reduced Trans-Activation, Impaired Sperm Production, and Male Infertility*. Vol. 82.
Wang, Ruey Sheng, Shuyuan Yeh, Lu Min Chen, Hung Yun Lin, Caixia Zhang, Jing Ni, Cheng Chia Wu, P. Anthony Di Sant’Agnese, Karen L. DeMesy-Bentley, Chii Ruey Tzeng, and Chawnshang Chang. 2006. “Androgen Receptor in Sertoli Cell Is Essential for Germ Cell Nursery and Junctional Complex Formation in Mouse Testes.” Endocrinology 147(12):5624–33. doi: 10.1210/en.2006-0138.
Welsh, M., L. Moffat, K. Belling, L. R. de França, T. M. Segatelli, P. T. K. Saunders, R. M. Sharpe, and L. B. Smith. 2012. “Androgen Receptor Signalling in Peritubular Myoid Cells Is Essential for Normal Differentiation and Function of Adult Leydig Cells.” International Journal of Andrology 35(1):25–40. doi: 10.1111/j.1365-2605.2011.01150.x.
Willems, Ariane, Sergio R. Batlouni, Arantza Esnal, Johannes V. Swinnen, Philippa T. K. Saunders, Richard M. Sharpe, Luiz R. França, Karel de Gendt, and Guido Verhoeven. 2010. “Selective Ablation of the Androgen Receptor in Mouse Sertoli Cells Affects Sertoli Cell Maturation, Barrier Formation and Cytoskeletal Development.” PLoS ONE 5(11). doi: 10.1371/journal.pone.0014168.
Wu, D. I., Grace Lin, and Andrea C. Gore. 2009. “Age-Related Changes in Hypothalamic Androgen Receptor and Estrogen Receptor in Male Rats.” The Journal of Comparative Neurology 512:688–701. doi: 10.1002/cne.21925.
Yu, I. Chen, Hung Yun Lin, Ning Chun Liu, Ruey Shen Wang, Janet D. Sparks, Shuyuan Yeh, and Chawnshang Chang. 2008. “Hyperleptinemia without Obesity in Male Mice Lacking Androgen Receptor in Adipose Tissue.” Endocrinology 149(5):2361–68. doi: 10.1210/en.2007-0516.
Yu, Shengqiang, Chiuan Ren Yeh, Yuanjie Niu, Hong Chiang Chang, Yu Chieh Tsai, Harold L. Moses, Chih Rong Shyr, Chawnshang Chang, and Shuyuan Yeh. 2012. “Altered Prostate Epithelial Development in Mice Lacking the Androgen Receptor in Stromal Fibroblasts.” Prostate 72(4):437–49. doi: 10.1002/pros.21445.
Zhang, Caixia, Shuyuan Yeh, Yen-Ta Chen, Cheng-Chia Wu, Kuang-Hsiang Chuang, Hung-Yun Lin, Ruey-Sheng Wang, Yu-Jia Chang, Chamindrani Mendis-Handagama, Liquan Hu, Henry Lardy, Chawnshang Chang, and † † George. 2006. Oligozoospermia with Normal Fertility in Male Mice Lacking the Androgen Receptor in Testis Peritubular Myoid Cells.
Zhou, Wei, Gensheng Wang, Christopher L. Small, Zhilin Liu, Connie C. Weng, Lizhong Yang, Michael D. Griswold, and Marvin L. Meistrich. 2011. “Erratum: Gene Expression Alterations by Conditional Knockout of Androgen Receptor in Adult Sertoli Cells of Utp14bjsd/Jsd (Jsd) Mice (Biology of Reproduction (2010) 83, (759-766) DOI: 10.1095/Biolreprod.110.085472).” Biology of Reproduction 84(2):400–408.
List of Non Adjacent Key Event Relationships
Relationship: 3488: Decrease, intratesticular testosterone leads to Hypospadias
AOPs Referencing Relationship
| AOP Name | Adjacency | Weight of Evidence | Quantitative Understanding |
|---|---|---|---|
| Decreased testosterone synthesis leading to hypospadias in male (mammalian) offspring | non-adjacent | Moderate |
Evidence Supporting Applicability of this Relationship
| Life Stage | Evidence |
|---|---|
| Foetal | High |
| Sex | Evidence |
|---|---|
| Male | High |
Taxonomic applicability
The development and differentiation of the penis is driven by androgen hormones, mainly produced by fetal testes, in all mammals. It is therefore biologically plausible that this KER is applicable to all mammals (Murashima et al., 2015). The empirical evidence in this KER provides support that reduced intratesticular testosterone levels in fetal life can cause hypospadias in rats. Studies in humans with gonadal dysgenesis and concurrent hypospadias support this KER’s applicability to humans (Boehmer et al., 2001; Crone et al., 2002).
Sex applicability
This KER is applicable to males, where the testes are the primary sex organ.
Life stage applicability
The genital tubercle is programmed by androgen hormones in the masculinization programming window (gestational days (GD) 16-20 in rats, and gestational weeks (GW) 8-14 in humans ), when the testes produce high levels of testosterone (Sharpe, 2020; Welsh et al., 2014). The genital tubercle starts differentiating in fetal life, and in humans the penis is fully formed at birth, where hypospadias is usually diagnosed (Yu et al., 2019). In rats and mice, penis development continues postnatally for around 20-25 days, and hypospadias is optimally diagnosed after this timepoint, although it may also be observed earlier (Schlomer et al., 2013; Sinclair et al., 2017).
Key Event Relationship Description
This non-adjacent KER describes a fetal decrease in testis testosterone leading to hypospadias in male offspring. In this KER, intratesticular testosterone levels can both be measured in whole testes homogenates or by measuring ex vivo testosterone production from cultured testes.
In male mammals, the testes differentiate in early fetal life and begin steroidogenesis to synthesize testosterone. Testosterone is secreted from the fetal testes for initiation of differentiation of the male reproductive tissues. Testosterone acts at the androgen receptor (AR) or is converted by 5α-reductase to the more potent androgen dihydrotestosterone (DHT). Activation of AR in the bipotential genital tubercle starts differentiation into a penis. While penis differentiation is a longer process, programming of the genital tubercle is largely constrained to a fixed period (GD 16-20 in rats, GW 8-14 in humans), when testicular testosterone production is high (Sharpe, 2020; Welsh et al., 2014). Failure of proper penis differentiation can cause genital malformations, of which the most common is hypospadias, where the urethral opening is on the underside of the penis.
A decrease in intratesticular testosterone levels may therefore lead to hypospadias in male offspring.
Evidence Supporting this KER
Biological PlausibilityThe biological plausibility for this KER is judged to be high given the canonical biological knowledge on normal reproductive development.
Differentiation of the penis is programmed during fetal development. Once the testes have formed around GW8 in humans and GD16 in rats, they synthesize testosterone through the steroidogenesis pathway (Murashima et al., 2015). Although the adrenal glands may also produce testosterone, the testes are the main site of testosterone production (Naamneh Elzenaty et al., 2022). Testosterone is secreted from the testes and is transported to the peripheral tissues, including the genital tubercle. Testosterone may act directly on the AR or be converted to the more potent androgen DHT.
The genital tubercle is the bipotential structure that upon hormonal cues differentiates to either penis or clitoris. Both human and rodent genital tubercles express AR (C. M. Amato & Yao, 2021; Baskin et al., 2020). Upon activation of AR, the genital tubercle differentiates to a penis by elongation and formation of a central urethra which terminates at the tip of the penis (C. Amato et al., 2022). The programming of the genital tubercle happens in the masculinization programming window (GD 16-20 in rats, GW 8-14 in humans) (Welsh et al., 2014), although elongation and growth of the penis is also programmed later, at least in rats (Welsh et al., 2008). Hypospadias is one of the most common genital malformations caused by disruptions to penis development (Baskin & Ebbers, 2006; Yu et al., 2019).
Given the dependency of testosterone for penis differentiation, either through direct AR activation or conversion to DHT, it is plausible that a decrease in intratesticular testosterone will cause hypospadias.
Empirical EvidenceThe empirical evidence from studies in animals for this KER is overall judged as moderate
From the data collection, three data sets were extracted. The data sets included different stressors causing reduced fetal levels of testosterone, all in rats (table 2 and appendix 2, 59p4izmm69_KER_3488_appendix_2.pdf). All studies showed concurrent hypospadias in the male offspring.
Table 2 Empirical evidence for KER 3488 LOAEL: Lowest observed adverse effect level; see appendix 2 for specifications: 59p4izmm69_KER_3488_appendix_2.pdf
|
Stressors(s) |
Effect on upstream event (intratesticular testosterone) |
Effect on downstream event hypospadias |
Reference |
|
|
Rat |
Dibutyl phthalate |
LOAEL 750 mg/kg bw/day |
LOAEL 750 mg/kg bw/day |
(van den Driesche et al., 2020) |
|
Rat |
Dibutyl phthalate |
LOAEL 500 mg/kg bw/day |
LOAEL 500 mg/kg bw/day |
(Drake et al., 2009) |
|
Rat |
Diisooctyl phthalate |
LOAEL 0.1 mg/kg bw/day |
LOAEL 1 mg/kg bw/day |
(Saillenfait et al., 2013) |
Supporting human studies
Supporting the empirical evidence are human cases of patients with less testicular tissue (i.e. partial gonadal dysgenesis) which can cause severe hypospadias (Boehmer et al., 2001; Crone et al., 2002).
Dose concordance
One study informs and supports dose concordance for this KER. In this study, diisocytol phthalate caused reduced ex vivo testosterone production at a dose of 0.1 mg/kg bw/day, while hypospadias was observed in male offspring at 1 mg/kg bw/day (Saillenfait et al., 2013).
Temporal concordance
Empirical evidence indirectly supports temporal concordance. In all studies, the exposure window was prenatal, and low testosterone levels were detected at GD18-22, while hypospadias was assessed in adult rats. There are, however, no assessments of hypospadias at earlier timepoints to establish when the phenotype became visible.
Incidence concordance
Incidence concordance cannot be directly informed from the empirical evidence, because testosterone levels are reported as means of all values and is a continuous variable. However, given that hypospadias was not registered in all males in any of the studies, this could suggest a higher incidence of lower testosterone levels than the incidence of hypospadias.
Uncertainties and InconsistenciesIn one study (Drake et al., 2009), testosterone levels were only reduced when performing statistical analysis on individual values and not on litter means. Hypospadias was observed in 30% of males. The difference in statistical significance between litter means and individual values is an uncertainty to this study.
In (Saillenfait et al., 2013), intratesticular testosterone was only measured in ex vivo testes cultures, which are assumed to be a good proxy for intratesticular testosterone levels, although it should be kept in mind as an uncertainty.
Another uncertainty for this KER from the literature is the observation that humans with 5α-reductase deficiency have hypospadias due to low DHT levels despite normal or higher testosterone levels (Mendonca et al., 1996). This indicates that the effects of low testosterone may be more through reduced conversion to DHT than due to a direct loss of testosterone action on AR. To this, there is also the existence of a “backdoor pathway” to DHT in humans. This pathway in peripheral tissues (i.e. not testes) can circumvent testosterone as a precursor for DHT by synthesis of DHT is from reduction of androsterone by 17β-HSD (Miller & Auchus, 2019). This would create the possibility that testosterone is not required for DHT production and ultimately AR activation.
Quantitative Understanding of the Linkage
The quantitative understanding of this KER is low.
Response-response relationshipA model for phthalate-induced malformations has been developed which aims to predict the frequency of hypospadias related to a phthalate’s reduction in ex vivo testosterone production. The model predicted that a 60% reduction in testosterone levels would induce hypospadias, although the predictivity of this model was not good when tested for one phthalate (Earl Gray et al., 2024).
Time-scaleThe time-scale of this KER largely depends on species, but is likely weeks. In humans, the masculinization programming window is weeks long, while in rodents it is days (Sharpe, 2020; Welsh et al., 2014). Hypospadias is diagnosed at birth in humans (Yu et al., 2019) and can also be observed at birth in rodents, but as development of the penis continues after birth in rodents, hypospadias may be more optimally evaluated later in juvenile or adult male rats (Schlomer et al., 2013; Sinclair et al., 2017).
Known modulating factorsThere are no known modulating factors for this KER
Known Feedforward/Feedback loops influencing this KERThere are no known feedback/feedforward loops for this KER.
References
Amato, C., Fricke, A., Marella, S., Mogus, J., Bereman, M., & McCoy, K. (2022). An experimental evaluation of the efficacy of perinatal sulforaphane supplementation to decrease the incidence and severity of vinclozolin-induced hypospadias in the mouse model. Toxicology and Applied Pharmacology, 451, 116177. https://doi.org/10.1016/j.taap.2022.116177
Amato, C. M., & Yao, H. H.-C. (2021). Developmental and sexual dimorphic atlas of the prenatal mouse external genitalia at the single-cell level. Proceedings of the National Academy of Sciences of the United States of America, 118(25). https://doi.org/10.1073/pnas.2103856118
Baskin, L., Cao, M., Sinclair, A., Li, Y., Overland, M., Isaacson, D., & Cunha, G. R. (2020). Androgen and estrogen receptor expression in the developing human penis and clitoris. Differentiation; Research in Biological Diversity, 111, 41–59. https://doi.org/10.1016/j.diff.2019.08.005
Baskin, L., & Ebbers, M. (2006). Hypospadias: Anatomy, etiology, and technique. Journal of Pediatric Surgery, 41(3), 463–472. https://doi.org/10.1016/j.jpedsurg.2005.11.059
Boehmer, A., Nijman, R., Lammers, B., de Coninck, S., Van Hemel, J., Themmen, A., Mureau, M., de Jong, F., Brinkmann, A., Niermeijer, M., & Drop, S. (2001). Etiological studies of severe or familial hypospadias. The Journal of Urology, 165(4), 1246–1254.
Crone, J., Amann, G., Gheradini, R., Kirchlechner, V., & Fékété, C. (2002). Management of 46, XY partial gonadal dysgenesis—Revisited. Wiener Klinische Wochenschrift, 114(12), 462–467.
Drake, A., van den Driesche, S., Scott, H., Hutchison, G., Seckl, J., & Sharpe, R. (2009). Glucocorticoids Amplify Dibutyl Phthalate-Induced Disruption of Testosterone Production and Male Reproductive Development. ENDOCRINOLOGY, 150(11), 5055–5064. https://doi.org/10.1210/en.2009-0700
Earl Gray, L. J., Lambright, C., Evans, N., Ford, J., & Conley, M. (2024). Using targeted fetal rat testis genomic and endocrine alterations to predict the effects of a phthalate mixture on the male reproductive tract. Current Research in Toxicology, 7, 100180. https://doi.org/10.1016/j.crtox.2024.100180
Holmer, M. L., Zilliacus, J., Draskau, M. K., Hlisníková, H., Beronius, A., & Svingen, T. (2024). Methodology for developing data-rich Key Event Relationships for Adverse Outcome Pathways exemplified by linking decreased androgen receptor activity with decreased anogenital distance. Reproductive Toxicology, 128, 108662. https://doi.org/10.1016/j.reprotox.2024.108662
Murashima, A., Kishigami, S., Thomson, A., & Yamada, G. (2015). Androgens and mammalian male reproductive tract development. Biochimica et Biophysica Acta (BBA) - Gene Regulatory Mechanisms, 1849(2), 163–170. https://doi.org/10.1016/j.bbagrm.2014.05.020
Naamneh Elzenaty, R., Du Toit, T., & Flück, C. E. (2022). Basics of androgen synthesis and action. Best Practice & Research Clinical Endocrinology & Metabolism, 36(4), 101665. https://doi.org/10.1016/j.beem.2022.101665
Saillenfait, A., Sabaté, J., Robert, A., Cossec, B., Roudot, A., Denis, F., & Burgart, M. (2013). Adverse effects of diisooctyl phthalate on the male rat reproductive development following prenatal exposure. Reproductive Toxicology (Elmsford, N.Y.), 42, 192–202. https://doi.org/10.1016/j.reprotox.2013.09.004
Schlomer, B. J., Feretti, M., Rodriguez, E. J., Blaschko, S., Cunha, G., & Baskin, L. (2013). Sexual differentiation in the male and female mouse from days 0 to 21: A detailed and novel morphometric description. The Journal of Urology, 190(4 Suppl), 1610–1617. https://doi.org/10.1016/j.juro.2013.02.3198
Sharpe, R. (2020). Androgens and the masculinization programming window: Human-rodent differences. Biochemical Society Transactions, 48(4), 1725–1735. https://doi.org/10.1042/BST20200200
Sinclair, A., Cao, M., Pask, A., Baskin, L., & Cunha, G. (2017). Flutamide-induced hypospadias in rats: A critical assessment. Differentiation; Research in Biological Diversity, 94, 37–57. https://doi.org/10.1016/j.diff.2016.12.001
van den Driesche, S., Shoker, S., Inglis, F., Palermo, C., Langsch, A., & Otter, R. (2020). Systematic comparison of the male reproductive tract in fetal and adult Wistar rats exposed to DBP and DINP in utero during the masculinisation programming window. Toxicology Letters, 335, 37–50. https://doi.org/10.1016/j.toxlet.2020.10.006
Welsh, M., Saunders, P., Fisken, M., Scott, H., Hutchison, G., Smith, L., & Sharpe, R. (2008). Identification in rats of a programming window for reproductive tract masculinization, disruption of which leads to hypospadias and cryptorchidism. The Journal of Clinical Investigation, 118(4), 1479–1490. https://doi.org/10.1172/JCI34241
Welsh, M., Suzuki, H., & Yamada, G. (2014). The masculinization programming window. Endocrine Development, 27, 17–27. https://doi.org/10.1159/000363609
Yu, X., Nassar, N., Mastroiacovo, P., Canfield, M., Groisman, B., Bermejo-Sánchez, E., Ritvanen, A., Kiuru-Kuhlefelt, S., Benavides, A., Sipek, A., Pierini, A., Bianchi, F., Källén, K., Gatt, M., Morgan, M., Tucker, D., Canessa, M. A., Gajardo, R., Mutchinick, O. M., … Agopian, A. J. (2019). Hypospadias Prevalence and Trends in International Birth Defect Surveillance Systems, 1980-2010. European Urology, 76(4), 482–490. https://doi.org/10.1016/j.eururo.2019.06.027
Relationship: 3350: Decrease, circulating testosterone levels leads to Hypospadias
AOPs Referencing Relationship
| AOP Name | Adjacency | Weight of Evidence | Quantitative Understanding |
|---|---|---|---|
| Decreased testosterone synthesis leading to hypospadias in male (mammalian) offspring | non-adjacent | Low |
Evidence Supporting Applicability of this Relationship
| Life Stage | Evidence |
|---|---|
| Foetal | High |
| Sex | Evidence |
|---|---|
| Male | High |
Taxonomic applicability
Sexual differentiation of the penis is an androgen-driven process in mammals, and it is therefore biologically plausible that this KER is applicable to all mammals (Murashima et al., 2015). The empirical evidence in this KER provides support that hypospadias in humans is associated with reduced circulating testosterone levels in early life. The two in vivo studies included in the empirical evidence support the applicability to rats.
Sex applicability
The empirical evidence in this KER supports that reduced circulating testosterone is linked to hypospadias in males. Females do have circulating testosterone, but in much lower concentrations than males (Vesper et al., 2015). Moreover, the term hypospadias is mainly used for malformation of the male external genitalia.
Life stage applicability
The genital tubercle is programmed by the surge in androgen hormones during the masculinization programming window is (gestational days (GD) 16-20 in rats, and gestational weeks (GW) 8-14 in humans ) (Sharpe, 2020; Welsh et al., 2014). In humans, the penis is fully formed at birth (Yu et al., 2019), while penis development continues postnatally around 20-25 days in rats and mice (Schlomer et al., 2013; Sinclair et al., 2017).
Key Event Relationship Description
This non-adjacent KER describes a decrease in circulating testosterone (often measured in serum or plasma) during the fetal male masculinization programming window leading to hypospadias in male offspring.
In male mammals, testosterone along with its more potent derivative dihydrotestosterone (DHT) drives male reproductive differentiation. Produced by the fetal testes, testosterone is transported through blood to the peripheral reproductive tissues to bind the androgen receptor (AR) or be converted to DHT (Murashima et al., 2015). Activation of AR in the genital tubercle directs its differentiation to a penis, and failure of this differentiation can lead to malformations, including hypospadias where the urethra terminates on the underside of the penis. The androgen programming of the genital tubercle is largely (but not fully) constrained to the masculinization programming window (GD 16-20 in rats, GW 8-14 in humans), when circulating testosterone levels are high (Sharpe, 2020; Welsh et al., 2014).
A decrease in circulating testosterone levels in the masculinization programming window can thus disrupt penis differentiation and cause hypospadias.
Evidence Supporting this KER
Biological PlausibilityThe biological plausibility for this KER is judged to be high given the canonical biological knowledge on normal reproductive development.
Sexual differentiation in males, the external genitalia, is initiated and programmed in fetal life. Once the testes have formed, they synthesize testosterone through the steroidogenesis pathway and secrete it into circulation. Testosterone is transported in the blood either as free testosterone or bound to albumin or sex-hormone binding globulin. Testosterone is produced from around GD15 in fetal rats and GW8 in humans, which is also the onset of when testosterone levels can be measured in circulation. In peripheral tissues, testosterone can be converted to the more potent androgen dihydrotestosterone (DHT) by the enzyme 5α-reductase. Both DHT and testosterone bind and activate the androgen receptor (AR) to program fetal tissues to differentiate along the male pathway (Murashima et al., 2015; Trost & Mulhall, 2016; Welsh et al., 2014).
The genital tubercle is the bipotential structure that upon hormonal cues differentiates to either penis or clitoris. Both human and rodent genital tubercles express AR (C. M. Amato & Yao, 2021; Baskin et al., 2020). Upon activation of AR, the genital tubercle differentiates to a penis by elongation and formation of a central urethra which terminates at the tip of the penis (C. Amato et al., 2022). The programming of the genital tubercle happens in the masculinization programming window (GD 16-20 in rats, GW 8-14 in humans) (Welsh et al., 2014), although elongation and growth of the penis is also programmed later, at least in rats (Welsh et al., 2008). Hypospadias is one of the most common genital malformations caused by disruptions to penis development (Baskin & Ebbers, 2006; Yu et al., 2019).
Given the dependency of testosterone for penis differentiation, either through direct AR activation or conversion to DHT, it is plausible that a decrease in circulating levels of testosterone will cause hypospadias.
Empirical EvidenceThe empirical evidence from studies in animals for this KER is judged as low overall.
From the data collection, two toxicity studies were extracted which reported a decrease in circulating testosterone levels in fetal male rats and measured hypospadias in male offspring. However, both studies were classified as not reliable (category 3). Due to the lack of any reliable studies for this KER, the evidence from the two unreliable data sets is included, although they are considered weak evidence for the KER.
In this first study (Li et al., 2017), rats were exposed to 0 or 750 mg/kg bw/day dibutylphthalate (DBP) from GD13-18. At GD19, serum testosterone and hypospadias were evaluated. At this stage, 43.6% of males exposed to DBP had hypospadias upon examination. In the hypospadias group, serum testosterone levels were 3.93 nmol/mL compared to 15.74 nmol/mL in control male rats. In exposed rats without hypospadias, serum testosterone levels were 5.89 nmol/mL. This study was categorized as unreliable, mainly due to the evaluation of hypospadias being on GD19, a timepoint at which the penis is not finished differentiating. This poses an uncertainty, in particular regarding the frequency of hypospadias reported in the exposure group.
The second study (Vo et al., 2009) was considered unreliable due to a too low reliability score (64.7) with some key information not reported. In this study, no major deficiencies were noted. Rats were exposed to 0, 10, 100, or 500 mg/kg bw/day diethylhexylphthalate (DEHP) from GD11-21. At GD21, plasma testosterone levels were reduced in males in the highest exposure group (0.53 ±0.16 ng/mL) compared to control males (1.56 ±0.84 ng/mL), but not in the other dose groups. Hypospadias was evaluated in male offspring at PND63, and hypospadias was registered in 23/100 male pups exposed in utero to 500 mg/kg bw/day. There was no hypospadias in the other groups.
Supporting human case studies
Supporting this KER are case studies of humans with hypospadias and a reduction in circulating testosterone levels.
Studies extracted from the literature search are summarized in table 2.
Table 2: Human case studies of hypospadias with reductions in circulating testosterone levels (measured postnatally).
|
Case |
Effect on upstream event (circulating testosterone) |
Effect on downstream event hypospadias |
Reference |
|
Subject with mutation in HSD17B3 |
Six weeks: 2.5 nmol/L (reference range 0-6.5 nmol/L) 10 years: 3.6 nmol/L (reference range: 9.0-31.0 nmol/L) |
Ambiguous genitalia / hypospadias |
(Al-Sinani et al., 2015) |
|
7 subjects (0-2 years) with perineal or penoscrotal hypospadias |
Testosterone levels < 2 ng/mL after hCG stimulation. In 2 subjects, testosterone response returned to normal |
Perineal or penoscrotal hypospadias |
(Allen & Griffin, 1984) |
|
18-year old subject |
Basal testosterone levels 0.8 ng/mL (normal range 2.8-18.5 ng/mL). hCG stimulated testosterone levels: 0.9 ng/mL |
Perineal hypospadias |
(Ammini et al., 1997) |
|
2 subjects (15 and 12 years old) with INHA mutations |
Testosterone levels: 0.68 and 1.48 ng/mL (normal range 2.4-9.5 ng/mL) |
Hypospadias |
(Arslan Ates et al., 2022) |
|
8 subjects |
hCG-stimulated testosterone levels in childhood < 3 ng/mL |
Proximal hypospadias |
(Blanc et al., 2011) |
|
Subject with unilateral vanishing testes syndrome |
Insufficient testosterone syndrome after hCG stimulation |
Severe hypospadias |
(Boehmer et al., 2001) |
|
Newborn subject |
Testosterone levels: 18 ng/dL (normal range 60-570 ng/dL) |
Perineal hypospadias |
(Dean et al., 1984) |
|
13 subjects (1/2-10 years) |
Low basal testosterone (0-0.34 ng/mL) and poor or absent response to hCG stimulation |
Penoscrotal, scrotal or perineal hypospadias |
(Iyengar et al., 1986) |
|
5 year old subject |
Testosterone response to hCG stimulation: 12-17 ng/dL (normal values 667 ng/dL) |
Perineal hypospadias |
(Kaufman et al., 1983) |
|
Three subjects (0-4 years) with NR5A1 mutations |
Low testosterone levels (basal 0.05-0.4 ng/mL; hCG stimulated 0.58-0.9 ng/mL) |
Penoscrotal hypospadias |
(Köhler et al., 2009) |
|
Subject with mutation in the gene encoding LH receptor |
No response to hCG stimulation (0.05 ng/mL) |
Severe hypospadias |
(Misrahi et al., 1997) |
|
2 subjects evaluated at puberty |
Decreased testosterone |
One mild and one severe hypospadias |
(Moriya et al., 2010) |
|
10-year old subject with HSD17B3 mutation |
Baseline testosterone: 59.2 ng/dL, hCG stimulated testosterone: 139.9 ng/dL (no reference values given) |
Hypospadias |
(Neocleous et al., 2012) |
|
24-year old subject |
Testosterone levels 0.3-0.4 µg/100 mL (no reference values given) |
Severe hypospadias |
(New, 1970) |
|
6-year old subject |
Low testosterone response to hCG stimulation |
Perineal hypospadias |
(Pang et al., 1983) |
|
Infant subject with CYP11A1 mutation |
Low testosterone response to hCG stimulation (0.7 nmol/L), but normal serum testosterone |
Penoscrotal hypospadias |
(Parajes et al., 2012) |
|
Infant subject with LHGCR mutation |
At birth: 0.24 ng/mL 10 weeks: 0.06 ng/mL 2 years: 0.02 ng/mL (normal range 0.03-0.52 ng/mL). No response in testosterone levels after hCG stimulation |
Perineal hypospadias |
(Richard et al., 2011) |
|
Subject with WT1 mutation |
Low testosterone levels: 1.02 ng/mL (no reference values given) |
Glandular hypospadias |
(Schumacher et al., 2008) |
|
Subject with NR5A1 mutation |
12 hours old: 0.9 nmol/L (low) 2 days old: <0.1 nmol/L (normal) In later years, slight increase at 9 years, then normal or decreased levels during teen years |
Perineal hypospadias |
(Teoli et al., 2023) |
|
Newborn with GPC3 mutation (Simpson-Golabi-Behmel syndrome) |
1 day old: 42 ng/dL (reference range 75-400 ng/dL) 20 days old: 55 ng/dL (reference range: 60-400 ng/dL) 26 days old after hCG stimulation: 130 ng/dL (reference range: 60-400 ng/dL) |
Midshaft hypospadias |
(Villarreal et al., 2013) |
|
Subject with MAMLD1 mutation |
2 months: 49 ng/dL (reference level: 196 ng/dL) |
Hypospadias |
(Yeste et al., 2022) |
Dose concordance
Dose concordance cannot be informed from the two in vivo studies, although the study (Vo et al., 2009) does not argue against dose concordance as the upstream and downstream events were measured at the same dose of DEHP.
Temporal concordance
The in vivo studies do not directly inform temporal concordance, but in (Vo et al., 2009) plasma testosterone levels were decreased at GD21, while hypospadias was diagnosed in adult rats (PND63), long after exposure was ceased. In (Li et al., 2017), hypospadias and testosterone levels were assessed/measured at the same time point (GD19), however this is known not to be an optimal time point for hypospadias diagnosis in rats as the penis is not finished developing.
Incidence concordance
The study with DBP supports incidence concordance, because the non-hypospadiac rats exposed to DBP also had lower serum testosterone levels compared to control rats at GD19 (Li et al., 2017). This is however only weak evidence, as hypospadias was not evaluated at an optimal time point.
Uncertainties and Inconsistencies
The uncertainties of the two in vivo studies have been discussed. Both were classified as unreliable in the evaluation of methodological reliability. Of the two studies, the study by (Li et al., 2017) is considered most uncertain due to the timepoint of hypospadias assessment. A study with in utero exposure to an 5α-reductase inhibitor disrupted genital tubercle development at GD19, but hypospadias was not observed at PND90, indicating that assessment prior to birth may not be true indications of postnatal outcomes (Iguchi et al., 1991). The deficiencies in (Vo et al., 2009) are less severe but overall poses an uncertainty to the study due to missing key information about study design.
The uncertainties in the human evidence mainly pertains to the fact that testosterone levels were not measured during fetal life but in newborn or juvenile males. Given that most cases involve genetic mutations, which occur early in embryonic life, it is highly likely that the effects of mutations on testosterone levels manifest early in fetal life. Another uncertainty with the human cases which include mutations is that it cannot be excluded that the hypospadias phenotype is caused by the low testosterone levels and not directly by genetic mutation.
Another uncertainty for this KER from the literature is the observation that humans with 5α-reductase deficiency have hypospadias due to low DHT levels despite normal or higher testosterone levels (Mendonca et al., 1996). This indicates that the effects of low testosterone may be more through reduced conversion to DHT than due to a direct loss of testosterone action on AR. To this, there is also the existence of a “backdoor pathway” to DHT in humans. This pathway in peripheral tissues (i.e. not testes) can circumvent testosterone as a precursor for DHT by synthesis of DHT is from reduction of androsterone by 17β-HSD (Miller & Auchus, 2019). This would create the possibility that testosterone is not required for DHT production and ultimately AR activation.
Quantitative Understanding of the Linkage
The quantitative understanding of this KER is low.
Time-scaleThe time-scale for this KER depends on species but is likely weeks. Testosterone is secreted from around GW8 in humans (GD16 in rats), marking the beginning of the masculinization programming window and programming of the genital tubercle. Hypospadias is diagnosed at birth in humans (Yu et al., 2019) and can also be observed at birth in rodents, but as development of the penis continues after birth in rodents, hypospadias may be more optimally evaluated later in juvenile or adult male rats (Schlomer et al., 2013; Sinclair et al., 2017).
Known modulating factorsThere are no known modulating factors for this KER.
Known Feedforward/Feedback loops influencing this KERThere are no known feedback/feedforward loops for this KER.
References
Allen, T., & Griffin, J. (1984). Endocrine studies in patients with advanced hypospadias. The Journal of Urology, 131(2), 310–314. https://doi.org/10.1016/s0022-5347(17)50360-2
Al-Sinani, A., Mula-Abed, W., Al-Kindi, M., Al-Kusaibi, G., Al-Azkawi, H., & Nahavandi, N. (2015). A Novel Mutation Causing 17-β-Hydroxysteroid Dehydrogenase Type 3 Deficiency in an Omani Child: First Case Report and Review of Literature. Oman Medical Journal, 30(2), 129–134. https://doi.org/10.5001/omj.2015.27
Amato, C., Fricke, A., Marella, S., Mogus, J., Bereman, M., & McCoy, K. (2022). An experimental evaluation of the efficacy of perinatal sulforaphane supplementation to decrease the incidence and severity of vinclozolin-induced hypospadias in the mouse model. Toxicology and Applied Pharmacology, 451, 116177. https://doi.org/10.1016/j.taap.2022.116177
Amato, C. M., & Yao, H. H.-C. (2021). Developmental and sexual dimorphic atlas of the prenatal mouse external genitalia at the single-cell level. Proceedings of the National Academy of Sciences of the United States of America, 118(25). https://doi.org/10.1073/pnas.2103856118
Ammini, A., Sharma, D., Gupta, R., Mohapatra, I., Kucheria, K., Kriplani, A., Takkar, D., Mitra, D., & Vijayaraghavan, M. (1997). Familial male pseudohermaphroditism. Indian Journal of Pediatrics, 64(3), 419–423. https://doi.org/10.1007/BF02845218
Arslan Ates, E., Eltan, M., Sahin, B., Gurpinar Tosun, G., Seven Menevse, T., Geckinli, B., Greenfield, A., Turan, S., Bereket, A., & Guran, T. (2022). Homozygosity for a novel INHA mutation in two male siblings with hypospadias, primary hypogonadism, and high-normal testicular volume. European Journal of Endocrinology, 186(5), K25–K31. https://doi.org/10.1530/EJE-21-1230
Baskin, L., Cao, M., Sinclair, A., Li, Y., Overland, M., Isaacson, D., & Cunha, G. R. (2020). Androgen and estrogen receptor expression in the developing human penis and clitoris. Differentiation; Research in Biological Diversity, 111, 41–59. https://doi.org/10.1016/j.diff.2019.08.005
Baskin, L., & Ebbers, M. (2006). Hypospadias: Anatomy, etiology, and technique. Journal of Pediatric Surgery, 41(3), 463–472. https://doi.org/10.1016/j.jpedsurg.2005.11.059
Blanc, T., Ayedi, A., El-Ghoneimi, A., Abdoul, H., Aigrain, Y., Paris, F., Sultan, C., Carel, J., & Léger, J. (2011). Testicular function and physical outcome in young adult males diagnosed with idiopathic 46 XY disorders of sex development during childhood. European Journal of Endocrinology, 165(6), 907–915. https://doi.org/10.1530/EJE-11-0588
Boehmer, A., Nijman, R., Lammers, B., de Coninck, S., Van Hemel, J., Themmen, A., Mureau, M., de Jong, F., Brinkmann, A., Niermeijer, M., & Drop, S. (2001). Etiological studies of severe or familial hypospadias. The Journal of Urology, 165(4), 1246–1254.
Dean, H., Shackleton, C., & Winter, J. (1984). Diagnosis and natural history of 17-hydroxylase deficiency in a newborn male. The Journal of Clinical Endocrinology and Metabolism, 59(3), 513–520. https://doi.org/10.1210/jcem-59-3-513
Holmer, M. L., Zilliacus, J., Draskau, M. K., Hlisníková, H., Beronius, A., & Svingen, T. (2024). Methodology for developing data-rich Key Event Relationships for Adverse Outcome Pathways exemplified by linking decreased androgen receptor activity with decreased anogenital distance. Reproductive Toxicology, 128, 108662. https://doi.org/10.1016/j.reprotox.2024.108662
Iguchi, T., Uesugi, Y., Takasugi, N., & Petrow, V. (1991). Quantitative analysis of the development of genital organs from the urogenital sinus of the fetal male mouse treated prenatally with a 5 alpha-reductase inhibitor. The Journal of Endocrinology, 128(3), 395–401. https://doi.org/10.1677/joe.0.1280395
Iyengar, J., Rohatgi, M., Menon, P., Mathews, A., Verma, I., & Bhargava, S. (1986). Clinical, cytogenetic & hormonal profile in extreme hypospadias with bilaterally descended testes. The Indian Journal of Medical Research, 83, 604–609.
Kaufman, F., Costin, G., Goebelsmann, U., Stanczyk, F., & Zachmann, M. (1983). Male pseudohermaphroditism due to 17,20-desmolase deficiency. The Journal of Clinical Endocrinology and Metabolism, 57(1), 32–36. https://doi.org/10.1210/jcem-57-1-32
Köhler, B., Lin, L., Mazen, I., Cetindag, C., Biebermann, H., Akkurt, I., Rossi, R., Hiort, O., Grüters, A., & Achermann, J. (2009). The spectrum of phenotypes associated with mutations in steroidogenic factor 1 (SF-1, NR5A1, Ad4BP) includes severe penoscrotal hypospadias in 46,XY males without adrenal insufficiency. European Journal of Endocrinology, 161(2), 237–242. https://doi.org/10.1530/EJE-09-0067
Li, X., Li, J., Zhang, Y., & Zhou, Y. (2017). Di-n-butyl phthalate induced hypospadias relates to autophagy in genital tubercle via the PI3K/Akt/mTOR pathway. Journal of Occupational Health, 59(1), 8–16. https://doi.org/10.1539/joh.16-0089-OA
Mendonca, B., Inacio, M., Costa, E., Arnhold, I., Silva, F., Nicolau, W., Bloise, W., Russel, D., & Wilson, J. (1996). Male pseudohermaphroditism due to steroid 5alpha-reductase 2 deficiency. Diagnosis, psychological evaluation, and management. Medicine, 75(2), 64–76. https://doi.org/10.1097/00005792-199603000-00003
Miller, W. L., & Auchus, R. J. (2019). The “backdoor pathway” of androgen synthesis in human male sexual development. PLoS Biology, 17(4), e3000198. https://doi.org/10.1371/journal.pbio.3000198
Misrahi, M., Meduri, G., Pissard, S., Bouvattier, C., Beau, I., Loosfelt, H., Jolivet, A., Rappaport, R., Milgrom, E., & Bougneres, P. (1997). Comparison of immunocytochemical and molecular features with the phenotype in a case of incomplete male pseudohermaphroditism associated with a mutation of the luteinizing hormone receptor. The Journal of Clinical Endocrinology and Metabolism, 82(7), 2159–2165. https://doi.org/10.1210/jcem.82.7.4039
Moriya, K., Mitsui, T., Tanaka, H., Nakamura, M., & Nonomura, K. (2010). Long-term outcome of pituitary-gonadal axis and gonadal growth in patients with hypospadias at puberty. The Journal of Urology, 184(4), 1610–1614. https://doi.org/10.1016/j.juro.2010.04.022
Murashima, A., Kishigami, S., Thomson, A., & Yamada, G. (2015). Androgens and mammalian male reproductive tract development. Biochimica et Biophysica Acta (BBA) - Gene Regulatory Mechanisms, 1849(2), 163–170. https://doi.org/10.1016/j.bbagrm.2014.05.020
Neocleous, V., Sismani, C., Shammas, C., Efstathiou, E., Alexandrou, A., Ioannides, M., Argyrou, M., Patsalis, P., Phylactou, L., & Skordis, N. (2012). Duplication of exons 3-10 of the HSD17B3 gene: A novel type of genetic defect underlying 17β-HSD-3 deficiency. GENE, 499(2), 250–255. https://doi.org/10.1016/j.gene.2012.03.031
New, M. (1970). Male pseudohermaphroditism due to 17 alpha-hydroxylase deficiency. The Journal of Clinical Investigation, 49(10), 1930–1941. https://doi.org/10.1172/JCI106412
Pang, S., Levine, L., Stoner, E., Opitz, J., Pollack, M., Dupont, B., & New, M. (1983). Nonsalt-losing congenital adrenal hyperplasia due to 3 beta-hydroxysteroid dehydrogenase deficiency with normal glomerulosa function. The Journal of Clinical Endocrinology and Metabolism, 56(4), 808–818. https://doi.org/10.1210/jcem-56-4-808
Parajes, S., Chan, A., But, W., Rose, I., Taylor, A., Dhir, V., Arlt, W., & Krone, N. (2012). Delayed diagnosis of adrenal insufficiency in a patient with severe penoscrotal hypospadias due to two novel P450 side-change cleavage enzyme (CYP11A1) mutations (p.R360W; p.R405X). European Journal of Endocrinology, 167(6), 881–885. https://doi.org/10.1530/EJE-12-0450
Richard, N., Leprince, C., Gruchy, N., Pigny, P., Andrieux, J., Mittre, H., Manouvrier, S., Lahlou, N., Weil, J., & Kottler, M. (2011). Identification by array-Comparative Genomic Hybridization (array-CGH) of a large deletion of luteinizing hormone receptor gene combined with a missense mutation in a patient diagnosed with a 46,XY disorder of sex development and application to prenatal diagnosis. Endocrine Journal, 58(9), 769–776. https://doi.org/10.1507/endocrj.k11e-119
Schlomer, B. J., Feretti, M., Rodriguez, E. J., Blaschko, S., Cunha, G., & Baskin, L. (2013). Sexual differentiation in the male and female mouse from days 0 to 21: A detailed and novel morphometric description. The Journal of Urology, 190(4 Suppl), 1610–1617. https://doi.org/10.1016/j.juro.2013.02.3198
Schumacher, V., Gueler, B., Looijeng, L., Becker, J., Amann, K., Engers, R., Dotsch, J., Stoop, H., Schulz, W., & Royer-Pokora, B. (2008). Characteristics of testicular dysgenesis syndrome and decreased expression of SRY and SOX9 in Frasier syndrome. Molecular Reproduction and Development, 75(9), 1484–1494. https://doi.org/10.1002/mrd.20889
Sharpe, R. (2020). Androgens and the masculinization programming window: Human-rodent differences. Biochemical Society Transactions, 48(4), 1725–1735. https://doi.org/10.1042/BST20200200
Sinclair, A., Cao, M., Pask, A., Baskin, L., & Cunha, G. (2017). Flutamide-induced hypospadias in rats: A critical assessment. Differentiation; Research in Biological Diversity, 94, 37–57. https://doi.org/10.1016/j.diff.2016.12.001
Teoli, J., Mallet, D., Renault, L., Gay, C., Labrune, E., Bretone, P., Giscard D’Estaing, S., Cuzin, B., Dijoud, F., Roucher-Boulez, F., & Plotton, I. (2023). Case Report: Longitudinal follow-up and testicular sperm extraction in a patient with a pathogenic NR5A1 (SF-1) frameshift variant: P.(Phe70Serfs*5). Frontiers in Endocrinology, 14, 1171822. https://doi.org/10.3389/fendo.2023.1171822
Trost, L. W., & Mulhall, J. P. (2016). Challenges in Testosterone Measurement, Data Interpretation, and Methodological Appraisal of Interventional Trials. The Journal of Sexual Medicine, 13(7), 1029–1046. https://doi.org/10.1016/j.jsxm.2016.04.068
Vesper, H. W., Wang, Y., Vidal, M., Botelho, J. C., & Caudill, S. P. (2015). Serum Total Testosterone Concentrations in the US Household Population from the NHANES 2011-2012 Study Population. Clinical Chemisty, 61(12), 1495–1504. https://doi.org/10.1373/clinchem.2015.245969
Villarreal, D., Villarreal, H., Paez, A., Peppas, D., Lynch, J., Roeder, E., & Powers, G. (2013). A Patient With a Unique Frameshift Mutation in GPC3, Causing Simpson-Golabi-Behmel Syndrome, Presenting With Craniosynostosis, Penoscrotal Hypospadias, and a Large Prostatic Utricle. AMERICAN JOURNAL OF MEDICAL GENETICS PART A, 161(12), 3121–3125. https://doi.org/10.1002/ajmg.a.36086
Vo, T., Jung, E., Dang, V., Jung, K., Baek, J., Choi, K., & Jeung, E. (2009). Differential effects of flutamide and di-(2-ethylhexyl) phthalate on male reproductive organs in a rat model. The Journal of Reproduction and Development, 55(4), 400–411. https://doi.org/10.1262/jrd.20220
Welsh, M., Saunders, P., Fisken, M., Scott, H., Hutchison, G., Smith, L., & Sharpe, R. (2008). Identification in rats of a programming window for reproductive tract masculinization, disruption of which leads to hypospadias and cryptorchidism. The Journal of Clinical Investigation, 118(4), 1479–1490. https://doi.org/10.1172/JCI34241
Welsh, M., Suzuki, H., & Yamada, G. (2014). The masculinization programming window. Endocrine Development, 27, 17–27. https://doi.org/10.1159/000363609
Yeste, D., Aguilar-Riera, C., Canestrino, G., Fernández-Alvarez, P., Clemente, M., & Camats-Tarruella, N. (2022). A New MAMLD1 Variant in an Infant With Microphallus and Hypospadias With Hormonal Pattern Suggesting Partial Hypogonadotropic Hypogonadism-Case Report. Frontiers in Endocrinology, 13, 884107. https://doi.org/10.3389/fendo.2022.884107
Yu, X., Nassar, N., Mastroiacovo, P., Canfield, M., Groisman, B., Bermejo-Sánchez, E., Ritvanen, A., Kiuru-Kuhlefelt, S., Benavides, A., Sipek, A., Pierini, A., Bianchi, F., Källén, K., Gatt, M., Morgan, M., Tucker, D., Canessa, M. A., Gajardo, R., Mutchinick, O. M., … Agopian, A. J. (2019). Hypospadias Prevalence and Trends in International Birth Defect Surveillance Systems, 1980-2010. European Urology, 76(4), 482–490. https://doi.org/10.1016/j.eururo.2019.06.027
Relationship: 2828: Decrease, AR activation leads to Hypospadias
AOPs Referencing Relationship
| AOP Name | Adjacency | Weight of Evidence | Quantitative Understanding |
|---|---|---|---|
| Androgen receptor (AR) antagonism leading to hypospadias in male (mammalian) offspring | non-adjacent | High | |
| Decreased testosterone synthesis leading to hypospadias in male (mammalian) offspring | non-adjacent | High | |
| 5α-reductase inhibition leading to hypospadias in male (mammalian) offspring | non-adjacent |
Evidence Supporting Applicability of this Relationship
| Life Stage | Evidence |
|---|---|
| Foetal | High |
| Sex | Evidence |
|---|---|
| Male | High |
Taxonomic applicability
In mammals, androgens are one of the primary drivers of penis differentiation. Hypospadias has been observed in several mammals, but most frequently reported in laboratory rodents and in humans (Chang et al., 2020; S. Wang & Zheng, 2025). In vivo studies in rats and mice show that in utero exposure to anti-androgenic chemicals can cause hypospadias in male offspring (see table 3). Many human case studies report boys born with hypospadias and associated deficiency in steroid hormone synthesis, 5α-reductase activity, or androgen receptor (AR) activity (see table 4).
The biologically plausible domain of applicability may extend beyond the empirical domain because androgen-controlled development of male external genitalia is evolutionary conserved in most mammals and, to some extent, also in other vertebrate classes (Gredler et al., 2014). Hypospadias can in principle occur in all animals that form a genital tubercle and have been observed in many domestic animal species including dog (Sonne et al., 2008; Switonski et al., 2018), cat (Nowacka-Woszuk et al., 2014), cattle (Murakami, 2008), sheep (Smith et al., 2012), and horse (De Lorenzi et al., 2010) as well as in wildlife species such as polar bear (Stamper et al., 1999), giraffe (Meuffels et al., 2020), and Tamar Wallaby (Leihy et al., 2011). The observed hypospadias in these animals is not, per se, linked to anti-androgenic exposure, which has only been sparsely investigated in other species than mice, rats, and humans. One study in monkeys did show hypospadias upon oral exposure to finasteride (Prahalada et al., 1997), and bicalutamide exposure induced hypospadias in guinea pigs (S. Wang et al., 2018). A study in rabbits exposed to procymidone did not find hypospadias in males (Inawaka et al., 2010). Another study in hyenas did also not find hypospadias in males after exposure to the anti-androgen finasteride (Drea et al., 1998), but it should be noted that the hyenas have a remarkable sexual development where penile growth occur in both females and males before androgen synthesis is initiated (Cunha et al., 2014) (the studies in hyena and rabbit were identified in our evidence collection but were judged as ‘unreliable’ and therefore not included as empirical evidence).
Sex applicability
The androgen receptor is expressed in the fetal genital tubercle of both females and males (Amato & Yao, 2021; Baskin et al., 2020), but hypospadias is primarily a term used for a malformation of the penis (Baskin & Ebbers, 2006), limiting the applicability of this KER to males.
Life stage applicability
Differentiation of the penis occurs during fetal life in the masculinization programming window (MPW) (GD 16-20 in rats, around gestational weeks 8-14 in humans), when androgen production is high (Welsh et al., 2008; C. Wolf et al., 2000a). In rats, exposure to anti-androgenic chemicals outside of, or in the late part of the MPW does not cause hypospadias or only to a low degree (Clark et al., 1993; van den Driesche et al., 2017; C. Wolf et al., 2000a), while exposure in the earlier (or full) MPW causes a higher frequency of hypospadias (depending on dose and chemical) (table 3). In humans, hypospadias can be diagnosed at birth (X. Yu et al., 2019), while in rodents, some parts of penis development occur postnatally (Schlomer et al., 2013; Sinclair et al., 2017). In these species, hypospadias may be observed at birth but is optimally diagnosed and severity classified weeks later. Given that disruptions to androgen programming takes place in fetal life, even though the AO is best detected postnatally, the life stage applicability is defined as fetal life.
Key Event Relationship Description
This non-adjacent KER describes a fetal decrease in androgen receptor (AR) activation in the genital tubercle causing hypospadias in male offspring, postnatally. During fetal development, androgens induce differentiation of the bipotential genital tubercle to a penis, including closure of the urethra. Androgens signal through AR and reduced fetal AR activation can therefore disrupt penis differentiation and lead to the genital malformation hypospadias. Reduced AR activation may happen both through reduced ligand availability (testosterone or dihydrotestosterone (DHT)) and by direct antagonism of AR (Amato et al., 2022; Mattiske & Pask, 2021).
The upstream KE ‘decrease, androgen receptor activation’ (KE 1614) refers to the in vivo event of overall reduction in AR activation. In this case, it therefore refers to a reduction in AR activation in the genital tubercle. Currently, decreased AR activation in mammals is only directly measured in vitro and not in vivo. Instead, indirect assessment of this KE may come from assays measuring AR antagonism, 5α-reductase activity (the enzyme converting testosterone to DHT), or decreased androgen levels (Draskau et al., 2024).
Evidence Supporting this KER
Biological PlausibilityThe biological plausibility for this KER is judged as high. This is largely based on canonical knowledge on normal reproductive development.
The penis originates from a sexually bipotential structure, the genital tubercle, which may differentiate to either a penis or a clitoris, depending on internal cues during fetal development. In males, the fetal testes produce large amounts of testosterone, which can subsequently be converted to the more potent androgen DHT by 5α-reductase in peripheral tissues. Testosterone and DHT both signal through AR in target tissues to initiate masculinization (Amato et al., 2022; Murashima et al., 2015). The critical developmental window for androgen programming of masculinization has been identified in rats as GD16-20, and is proposed to be gestational weeks 8-14 in humans (Sharpe, 2020; Welsh et al., 2008). As part of the masculinization process orchestrated by androgens, the genital tubercle differentiates to a penis, which at this point expresses AR in both humans and rodents (Amato & Yao, 2021; Baskin et al., 2020). This includes androgen-mediated elongation of the tubercle, formation of the prepuce, and tubular internalization of urethra, which is closed at the distal tip of the glans penis (Amato et al., 2022). Failure of full closure of the urethra can result in hypospadias, in which the urethra terminates at the ventral side of the penis instead of at the tip (Baskin & Ebbers, 2006; Cohn, 2011).
The dependency of androgens for penile development has been demonstrated in mice with conditional or full knockout of Ar, which results in partly or full sex-reversal of males, including a female-like urethral opening (Willingham et al., 2006; Yucel et al., 2004; Zheng et al., 2015). Similarly, female rats and mice exposed in utero to testosterone present with varying degrees of intersexuality, including, in some cases, a penis (Greene & Ivy, 1937; Zheng et al., 2015).
Empirical EvidenceThe empirical evidence for this KER is generally judged high. This includes evidence from in vivo animal studies and evidence from studies in humans. The upstream KE ‘Decreased AR activity’ refers to an in vivo effect, for which no methods for measurement of this in vivo in mammals currently exist. The effects on the upstream KE were therefore indirectly informed as described in each section.
Animal studies
Effects on the upstream KE were indirectly informed by including animal studies with stressors that are known to reduce AR activity through antagonizing the AR, lowering testosterone production, or inhibiting 5α-reductase. Six stressors, with established anti-androgenic effects, were included (more detailed evaluation of these chemicals can be found in KER-2820 (Holmer et al., 2024)). Table 3 summarizes the empirical evidence and confidence level for each chemical. Details on included evidence is presented in Table 1 in Appendix 2, 9prbqyba2x_Appendix_2_KER_2828.pdf. In summary, all six substances were shown to cause hypospadias in male offspring, and the confidence level for all substances was judged as strong, as conflicting results could be explained (see the section ‘Uncertainties and inconsistencies’). Thus, antagonism or AR, inhibition of 5α-reductase, or reduction in testosterone synthesis, all lead to hypospadias.
Table 3 Summary of empirical evidence for the KER – animal studies. See Table 1 in Appendix 2 ( 9prbqyba2x_Appendix_2_KER_2828.pdf) for details.
|
Chemical |
Upstream effect |
Downstream effect |
Overall confidence |
|
Flutamide |
Androgen receptor antagonist (Simard et al., 1986). |
In utero exposure causes hypospadias in rat and mouse |
Strong |
|
Dibutyl phthalate (DBP)
|
Has been shown to reduce fetal intratesticular testosterone and serum testosterone in vivo, but exact mechanism is unknown (Foster, 2006). |
In utero exposure causes hypospadias in rat |
Strong |
|
Vinclozolin |
AR antagonist (Kelce et al., 1994, 1997) |
In utero exposure causes hypospadias in rat and mouse |
Strong |
|
Di(2-ethylhexyl) phthalate (DEHP) |
Has been shown to reduce fetal intratesticular testosterone and serum testosterone in vivo, but exact mechanism is unknown (Parks et al., 2000). |
In utero exposure causes hypospadias in rat |
Strong |
|
Procymidone |
AR antagonist (Ostby et al., 1999). |
In utero exposure causes hypospadias in rat |
Strong |
|
Finasteride |
5α-reductase inhibitor, causing a reduction in DHT (Rittmaster & Wood, 1994). |
In utero exposure causes hypospadias in rat |
Strong |
Supporting human evidence
Effects on the upstream KE were indirectly informed by including studies in humans with a condition (genetic or other) that would reduce or disrupt either 1) function of AR, 2) conversion of testosterone to DHT by disrupting 5α-reductase activity, or 3) production of androgen hormones. Studies measuring low testosterone levels with no underlying cause were also included (see evidence collection strategy). Table 4 lists the studies, in which these conditions were linked to hypospadias in males.
Table 4 Supporting evidence for the KER – human studies. The table lists human studies reporting hypospadias in association with an upstream defect in AR activity, grouped according to the precise effect, and how it was diagnosed (mutation, in vitro activity, or blood hormone and metabolite profile). SRD5A2: 5α-reductase 2; HSD17B3: 17β-hydroxysteroid dehydrogenase 3; HSD3B2: 3β-hydroxysteroid dehydrogenase 2; CYP17A1: 17α-hydroxylase. See table 2 in Appendix 2 (9prbqyba2x_Appendix_2_KER_2828.pdf) for all included references.
|
Effect on upstream KE |
Supporting studies |
|
Effects on Androgen receptor |
|
|
AR mutations |
27 studies |
|
Extended CAG repeat length in AR |
4 studies |
|
Reduced AR activity (e.g. low receptor binding) in in vitro genital skin fibroblasts |
11 studies |
|
Effects on 5α-reductase activity |
|
|
SRD5A2 mutations |
30 studies |
|
SRD5A2 deficiency, diagnosed by T/DHT-ratio and/or reduced in vitro 5α-reductase activity in genital skin fibroblasts |
8 studies |
|
Effects on upstream steroidogenesis enzymes |
|
|
HSD17B3 mutations |
6 studies |
|
HSD3B2 mutations |
5 studies |
|
CYP17A1 mutation |
1 study |
|
HSD17B3 deficiency, diagnosed by hormone and metabolite profile |
2 studies |
|
HSD3B2 deficiency, diagnosed by hormone and metabolite profile |
4 studies |
|
CYP17A1 deficiency, diagnosed by hormone and metabolite profile |
5 studies |
|
Other upstream effects on low testosterone |
|
|
Low testosterone due to gonadal dysgenesis or hypogonadism |
7 studies |
|
Low basal testosterone or low testosterone response to hCG stimulation. Idiopathic or rare mutations. |
7 studies |
Six case-control studies were extracted, all of which found a correlation between lower testosterone levels (basal or hCG-stimulated) and hypospadias (Austin et al., 2002; Okuyama et al., 1981; Raboch et al., 1976; Ratan et al., 2012; Svensson et al., 1979; Yadav et al., 2011). In two of these studies, the correlation was age-dependent (Austin et al., 2002; Raboch et al., 1976)
One epidemiologic study was extracted, which investigated the association between phthalate exposure and hypospadias risk. Western Australian women exposed through their occupation to phthalates were more likely to have sons with hypospadias (Nassar et al., 2010). It should be noted that there are reported species differences in the effects of phthalates (including DEHP and DBP) on fetal testosterone production between humans, mice, and rats, and the direct translatability of the in vivo evidence is uncertain (Sharpe, 2020).
Dose concordance
Direct information about dose concordance is not available because AR activity currently cannot be measured in vivo.
Indirect information on dose concordance can be obtained from empirical evidence. In utero exposure of rats to DBP caused a dose-dependent decrease in serum testosterone levels at PND70 with LOAEL 250 mg/kg bw/day. Hypospadias was observed at this stage with LOAEL 500 mg/kg bw/day (Jiang et al., 2007). It should be noted that fetal testosterone levels were not measured.
Temporal concordance
Direct information about temporal concordance is not available because AR activity currently cannot be measured in vivo.
Indirect information on temporal concordance can be obtained from empirical evidence. In two studies, in which rats were exposed in utero to 750 mg/kg bw/day DBP, intratesticular testosterone levels were reduced in fetal testes, while hypospadias was identified in adult males. Plasma levels of testosterone were also measured in adults, and testosterone levels in exposed males were not significantly different from control males (van den Driesche et al., 2017, 2020). This has also been shown in a study with 500 mg/kg bw/day DBP (Drake et al., 2009). These studies indicate temporal concordance. Another study with DBP-induced hypospadias in rats saw a dose-dependent reduction in serum testosterone levels at PND70 after in utero exposure to as low as 250 mg/kg bw/day from GD14-18 (Jiang et al., 2007), though fetal testosterone levels were not measured in this study.
Incidence Concordance
Direct information about dose concordance is not available because AR activity currently cannot be measured in vivo.
Indirect information on incidence concordance can be obtained from empirical evidence. In the dose-response study with DBP, the incidence of hypospadias was 6.8% for 500 mg/kg bw/day DBP and 41.3% for 750 mg/kg bw/day. When separating hypospadias males from exposed males without hypospadias, plasma testosterone levels were decreased in both groups, indicating that DBP reduced testosterone levels at higher incidence than hypospadias (Jiang et al., 2007). The same was seen in another study with DBP, in which serum testosterone levels at PND7 were reduced in both hypospadiac and non-malformed males exposed to 750 mg/kg bw/day DBP from GD14-18 (Jiang et al., 2016).
Uncertainties and InconsistenciesThe in vivo studies do not directly inform about the upstream KE, ‘decreased AR activity’. The direct concordance between the KEs can therefore not be determined from the evidence.
For flutamide, two studies reported 100% hypospadias frequencies at doses of 6.25 and 10 mg/kg bw/day (Goto et al., 2004; McIntyre et al., 2001), while another study found a frequency of 56.9% when giving 20 mg/kg bw/day (Kita et al., 2016). This might be explained by a longer exposure window in the first two studies and uncertainties in assessment of hypospadias.
For DBP, there were discrepancies in whether 250 mg/kg bw/day was LOAEL (Mylchreest et al., 1998, 1999) or NOAEL (Jiang et al., 2007) for DBP. This conflict was explained by differences in exposure windows, supported by the observation that the frequency of hypospadias at 250 mg/kg bw/day was reported as very low (Mylchreest et al., 1998, 1999).
One study with vinclozolin (Ostby J et al., 1999) and one with procymidone (Hass et al., 2012) did not find hypospadias after in utero exposure. In both cases, this was likely due to too low doses tested.
In most of the human studies of steroidogenesis deficiency, serum or plasma levels of testosterone were reduced at baseline and/or upon hCG stimulation (Al-Sinani et al., 2015; Ammini et al., 1997; Cara et al., 1985; Chen, Huang, et al., 2021; Dean et al., 1984; Galli-Tsinopoulou et al., 2018; Imperato-McGinley et al., 1979; Kaufman et al., 1983; Mendonca et al., 1987, 2000; Neocleous et al., 2012; New, 1970; Pang et al., 1983; Perrone et al., 1985; Rabbani et al., 2012; Sherbet et al., 2003), but in a few studies, testosterone levels were normal (Donadille et al., 2018; Kon et al., 2015; Luna et al., 2021). In these cases, the effect of these deficiencies on tissue AR activity is uncertain.
For AR CAG repeat length, a case-control study did not find an association with hypospadias (Radpour R et al., 2007), but this could be because the hypospadias cases included had other etiologies.
Lastly, as there are currently no universal guidelines for identification and scoring of hypospadias in rodents, there are large variations in methods of assessment, and minor cases of hypospadias may be overlooked in some studies and included in others. This poses an uncertainty in the frequency reports in the scientific evidence.
Quantitative Understanding of the Linkage
The quantitative understanding of the relationship is low. As there are currently no direct measurement methods of the upstream KE (reduced AR activity) in mammals, quantification of the relationship is difficult to assess.
Response-response relationshipA model for phthalates has been developed, aiming to predict the frequency of hypospadias in male offspring based on reductions in ex vivo testosterone production, an indirect indication of AR activity. In this model, hypospadias was induced from around a 60% reduction in testosterone levels. The model does not consider hypospadias severity and is only for phthalate chemicals (Earl Gray et al., 2024).
Time-scaleThe time-scale of this KER depends on the species but is likely days to weeks.
AR activation happens within minutes, from ligand binding to nuclear translocation and promotor activation (Nightingale et al., 2003; Schaufele et al., 2005), while transcriptional and translational effects are observed minutes to hours later (Kang et al., 2002). AR programming of the genital tubercle occurs during fetal development in the Masculinization Programming Window (Sharpe, 2020). The time-scale for morphological effects in the tissue then depends on the species. In humans, penis development is completed prior to birth and hypospadias can be observed at birth. In rodents, penis development is not fully completed until weeks after birth, but hypospadias can often be observed earlier than this (table 3).
Known modulating factors|
Modulating Factors |
MF details |
Effects on the KER |
References |
|
AR CAG repeat length |
Extended CAG repeat length in AR is associated with reduced AR activity |
Higher risk of hypospadias development |
(Chamberlain et al., 1994) |
There are no known feedforward/feedback loops influencing this KER.
References
Al-Sinani, A., Mula-Abed, W., Al-Kindi, M., Al-Kusaibi, G., Al-Azkawi, H., & Nahavandi, N. (2015). A Novel Mutation Causing 17-β-Hydroxysteroid Dehydrogenase Type 3 Deficiency in an Omani Child: First Case Report and Review of Literature. Oman Medical Journal, 30(2), 129–134. https://doi.org/10.5001/omj.2015.27
Amato, C. M., & Yao, H. H.-C. (2021). Developmental and sexual dimorphic atlas of the prenatal mouse external genitalia at the single-cell level. Proceedings of the National Academy of Sciences of the United States of America, 118(25). https://doi.org/10.1073/pnas.2103856118
Amato, C. M., Yao, H. H.-C., & Zhao, F. (2022). One Tool for Many Jobs: Divergent and Conserved Actions of Androgen Signaling in Male Internal Reproductive Tract and External Genitalia. Frontiers in Endocrinology, 13, 910964. https://doi.org/10.3389/fendo.2022.910964
Ammini, A., Sharma, D., Gupta, R., Mohapatra, I., Kucheria, K., Kriplani, A., Takkar, D., Mitra, D., & Vijayaraghavan, M. (1997). Familial male pseudohermaphroditism. Indian Journal of Pediatrics, 64(3), 419–423. https://doi.org/10.1007/BF02845218
Austin, P., Siow, Y., Fallat, M., Cain, M., Rink, R., & Casale, A. (2002). The relationship between müllerian inhibiting substance and androgens in boys with hypospadias. The Journal of Urology, 168(4), 1784–1788; discussion 1788. https://doi.org/10.1097/01.ju.0000023680.64155.5c
Baskin, L., Cao, M., Sinclair, A., Li, Y., Overland, M., Isaacson, D., & Cunha, G. R. (2020). Androgen and estrogen receptor expression in the developing human penis and clitoris. Differentiation; Research in Biological Diversity, 111, 41–59. https://doi.org/10.1016/j.diff.2019.08.005
Baskin, L., & Ebbers, M. (2006). Hypospadias: Anatomy, etiology, and technique. Journal of Pediatric Surgery, 41(3), 463–472. https://doi.org/10.1016/j.jpedsurg.2005.11.059
Cara, J., Jr Moshang, T., Bongiovanni, A., & Marx, B. (1985). Elevated 17-hydroxyprogesterone and testosterone in a newborn with 3-beta-hydroxysteroid dehydrogenase deficiency. The New England Journal of Medicine, 313(10), 618–621. https://doi.org/10.1056/NEJM198509053131007
Chamberlain, N. L., Driver, E. D., & Miesfeld, R. L. (1994). The length and location of CAG trinucleotide repeats in the androgen receptor N-terminal domain affect transactivation function. Nucleic Acids Research, 22(15), 3181–3186. https://doi.org/10.1093/nar/22.15.3181
Chang, J., Wang, S., & Zheng, Z. (2020). Etiology of Hypospadias: A Comparative Review of Genetic Factors and Developmental Processes Between Human and Animal Models. Research and Reports in Urology, Volume 12, 673–686. https://doi.org/10.2147/RRU.S276141
Chen, L., Huang, H., Zhang, H., Zhu, G., & Zhu, M. (2021). Three cases of 3β-hydroxysteroid dehydrogenase deficiency: Clinical analysis. Advances in Clinical and Experimental Medicine : Official Organ Wroclaw Medical University, 30(3), 289–299. https://doi.org/10.17219/acem/131220
Clark, R., Anderson, C., Prahalada, S., Robertson, R., Lochry, E., Leonard, Y., Stevens, J., & Hoberman, A. (1993). Critical developmental periods for effects on male rat genitalia induced by finasteride, a 5 alpha-reductase inhibitor. Toxicology and Applied Pharmacology, 119(1), 34–40. https://doi.org/10.1006/taap.1993.1041
Cohn, M. J. (2011). Development of the external genitalia: Conserved and divergent mechanisms of appendage patterning. Developmental Dynamics, 240(5), 1108–1115. https://doi.org/10.1002/dvdy.22631
Cunha, G. R., Risbridger, G., Wang, H., Place, N. J., Grumbach, M., Cunha, T. J., Weldele, M., Conley, A. J., Barcellos, D., Agarwal, S., Bhargava, A., Drea, C., Hammond, G. L., Siiteri, P., Coscia, E. M., McPhaul, M. J., Baskin, L. S., & Glickman, S. E. (2014). Development of the external genitalia: Perspectives from the spotted hyena (Crocuta crocuta). Differentiation, 87(1–2), 4–22. https://doi.org/10.1016/j.diff.2013.12.003
De Lorenzi, L., Genualdo, V., Iannuzzi, A., Di Meo, G. P., Perucatti, A., Mancuso, R., Russo, M., Di Berardino, D., Parma, P., & Iannuzzi, L. (2010). Cytogenetic and Genetic Studies in a Hypospadic Horse (Equus caballus, 2n = 64). Sexual Development, 4(6), 352–357. https://doi.org/10.1159/000319527
Dean, H., Shackleton, C., & Winter, J. (1984). Diagnosis and natural history of 17-hydroxylase deficiency in a newborn male. The Journal of Clinical Endocrinology and Metabolism, 59(3), 513–520. https://doi.org/10.1210/jcem-59-3-513
Donadille, B., Houang, M., Netchine, I., Siffroi, J., & Christin-Maitre, S. (2018). Human 3beta-hydroxysteroid dehydrogenase deficiency associated with normal spermatic numeration despite a severe enzyme deficit. Endocrine Connections, 7(3), 395–402. https://doi.org/10.1530/EC-17-0306
Drake, A., van den Driesche, S., Scott, H., Hutchison, G., Seckl, J., & Sharpe, R. (2009). Glucocorticoids amplify dibutyl phthalate-induced disruption of testosterone production and male reproductive development. Endocrinology, 150(11), 5055–5064. https://doi.org/10.1210/en.2009-0700
Draskau, M. K., Rosenmai, A. K., Bouftas, N., Johansson, H. K. L., Panagiotou, E. M., Holmer, M. L., Elmelund, E., Zilliacus, J., Beronius, A., Damdimopolou, P., van Duursen, M., & Svingen, T. (2024). AOP Report: An Upstream Network for Reduced Androgen Signaling Leading to Altered Gene Expression of Androgen Receptor-Responsive Genes in Target Tissues. Environmental Toxicology and Chemistry. https://doi.org/10.1002/etc.5972
Drea, C., Weldele, M., Forger, N., Coscia, E., Frank, L., Licht, P., & Glickman, S. (1998). Androgens and masculinization of genitalia in the spotted hyaena (Crocuta crocuta). 2. Effects of prenatal anti-androgens. Journal of Reproduction and Fertility, 113(1), 117–127. https://doi.org/10.1530/jrf.0.1130117
Earl Gray, L. J., Lambright, C., Evans, N., Ford, J., & Conley, M. (2024). Using targeted fetal rat testis genomic and endocrine alterations to predict the effects of a phthalate mixture on the male reproductive tract. Current Research in Toxicology, 7, 100180. https://doi.org/10.1016/j.crtox.2024.100180
Foster, P. M. D. (2006). Disruption of reproductive development in male rat offspring following in utero exposure to phthalate esters. International Journal of Andrology, 29(1), 140–147. https://doi.org/10.1111/j.1365-2605.2005.00563.x
Galli-Tsinopoulou, A., Serbis, A., KotanidouP, E., Litou, E., Dokousli, V., Mouzaki, K., Fanis, P., Neocleous, V., & Skordis, N. (2018). 46,XY Disorder of Sex Development due to 17-Beta Hydroxysteroid Dehydrogenase Type 3 Deficiency in an Infant of Greek Origin. Journal of Clinical Research in Pediatric Endocrinology, 10(1), 74–78. https://doi.org/10.4274/jcrpe.4829
Goto, K., Koizumi, K., Takaori, H., Fujii, Y., Furuyama, Y., Saika, O., Suzuki, H., Saito, K., & Suzuki, K. (2004). Effects of flutamide on sex maturation and behavior of offspring born to female rats treated during late pregnancy. The Journal of Toxicological Sciences, 29(5), 517–534. https://doi.org/10.2131/jts.29.517
Gredler, M. L., Larkins, C. E., Leal, F., Lewis, A. K., Herrera, A. M., Perriton, C. L., Sanger, T. J., & Cohn, M. J. (2014). Evolution of External Genitalia: Insights from Reptilian Development. Sexual Development, 8(5), 311–326. https://doi.org/10.1159/000365771
Greene, R. R., & Ivy, A. C. (1937). THE EXPERIMENTAL PRODUCTION OF INTERSEXUALITY IN THE FEMALE RAT WITH TESTOSTERONE. Science (New York, N.Y.), 86(2226), 200–201. https://doi.org/10.1126/science.86.2226.200-a
Hass, U., Boberg, J., Christiansen, S., Jacobsen, P., Vinggaard, A., Taxvig, C., Poulsen, M., Herrmann, S., Jensen, B., Petersen, A., Clemmensen, L., & Axelstad, M. (2012). Adverse effects on sexual development in rat offspring after low dose exposure to a mixture of endocrine disrupting pesticides. Reproductive Toxicology (Elmsford, N.Y.), 34(2), 261–274. https://doi.org/10.1016/j.reprotox.2012.05.090
Holmer, M. L., Zilliacus, J., Draskau, M. K., Hlisníková, H., Beronius, A., & Svingen, T. (2024). Methodology for developing data-rich Key Event Relationships for Adverse Outcome Pathways exemplified by linking decreased androgen receptor activity with decreased anogenital distance. Reproductive Toxicology, 128, 108662. https://doi.org/10.1016/j.reprotox.2024.108662
Imperato-McGinley, J., Peterson, R., Stoller, R., & Goodwin, W. (1979). Male pseudohermaphroditism secondary to 17 beta-hydroxysteroid dehydrogenase deficiency: Gender role change with puberty. The Journal of Clinical Endocrinology and Metabolism, 49(3), 391–395. https://doi.org/10.1210/jcem-49-3-391
Inawaka, K., Kishimoto, N., Higuchi, H., & Kawamura, S. (2010). Maternal exposure to procymidone has no effects on fetal external genitalia development in male rabbit fetuses in a modified developmental toxicity study. JOURNAL OF TOXICOLOGICAL SCIENCES, 35(3), 299–307. https://doi.org/10.2131/jts.35.299
Jiang, J., Ma, L., Yuan, L., Wang, X., & Zhang, W. (2007). Study on developmental abnormalities in hypospadiac male rats induced by maternal exposure to di-n-butyl phthalate (DBP). Toxicology, 232(3), 286–293. https://doi.org/10.1016/j.tox.2007.01.018
Jiang, J., Zhong, C., Zhu, Y., XuL, D., Wood, K., Sun, W., Li, E., Liu, Z., Zhao, W., Ruan, Y., & Xia, S. (2016). Prenatal exposure to di-n-butyl phthalate (DBP) differentially alters androgen cascade in undeformed versus hypospadiac male rat offspring. Reproductive Toxicology (Elmsford, N.Y.), 61, 75–81. https://doi.org/10.1016/j.reprotox.2016.02.016
Kang, H.-Y., Huang, K.-E., Chang, S. Y., Ma, W.-L., Lin, W.-J., & Chang, C. (2002). Differential Modulation of Androgen Receptor-mediated Transactivation by Smad3 and Tumor Suppressor Smad4. Journal of Biological Chemistry, 277(46), 43749–43756. https://doi.org/10.1074/jbc.M205603200
Kaufman, F., Costin, G., Goebelsmann, U., Stanczyk, F., & Zachmann, M. (1983). Male pseudohermaphroditism due to 17,20-desmolase deficiency. The Journal of Clinical Endocrinology and Metabolism, 57(1), 32–36. https://doi.org/10.1210/jcem-57-1-32
Kelce, W. R., Lambright, C. R., Gray, L. E., & Roberts, K. P. (1997). Vinclozolin andp,p′-DDE Alter Androgen-Dependent Gene Expression:In VivoConfirmation of an Androgen Receptor-Mediated Mechanism. Toxicology and Applied Pharmacology, 142(1), 192–200. https://doi.org/10.1006/taap.1996.7966
Kelce, W. R., Monosson, E., Gamcsik, M. P., Laws, S. C., & Gray, L. E. (1994). Environmental Hormone Disruptors: Evidence That Vinclozolin Developmental Toxicity Is Mediated by Antiandrogenic Metabolites. Toxicology and Applied Pharmacology, 126(2), 276–285. https://doi.org/10.1006/taap.1994.1117
Kita, D., Meyer, K., Venturelli, A., Adams, R., Machado, D., Morais, R., Swan, S., Gennings, C., & Martino-Andrade, A. (2016). Manipulation of pre and postnatal androgen environments and anogenital distance in rats. TOXICOLOGY, 368, 152–161. https://doi.org/10.1016/j.tox.2016.08.021
Kon, M., Suzuki, E., Dung, V., Hasegawa, Y., Mitsui, T., Muroyas, K., Ueoka, K., Igarashi, N., Nagasaki, K., Oto, Y., Hamajima, T., Yoshino, K., Igarashi, M., Kato-Fukui, Y., Nakabayashi, K., Hayashi, K., Hata, K., Matsubara, Y., Moriya, K., … Fukami, M. (2015). Molecular basis of non-syndromic hypospadias: Systematic mutation screening and genome-wide copy-number analysis of 62 patients. HUMAN REPRODUCTION, 30(3), 499–506. https://doi.org/10.1093/humrep/deu364
Leihy, M. W., Shaw, G., Wilson, J. D., & Renfree, M. B. (2011). Development of the Penile Urethra in the Tammar Wallaby. Sexual Development, 5(5), 241–249. https://doi.org/10.1159/000334053
Luna, S., Wegner, D., Gale, S., Yang, P., Hollander, A., St Dennis-Feezle, L., Nabhan, Z., Ory, D., Cole, F., & Wambach, J. (2021). Whole exome sequencing and functional characterization increase diagnostic yield in siblings with a 46, XY difference of sexual development (DSD). The Journal of Steroid Biochemistry and Molecular Biology, 212, 105908. https://doi.org/10.1016/j.jsbmb.2021.105908
Mattiske, D. M., & Pask, A. J. (2021). Endocrine disrupting chemicals in the pathogenesis of hypospadias; developmental and toxicological perspectives. Current Research in Toxicology, 2, 179–191. https://doi.org/10.1016/j.crtox.2021.03.004
McIntyre, B., Barlow, N., & Foster, P. (2001). Androgen-mediated development in male rat offspring exposed to flutamide in utero: Permanence and correlation of early postnatal changes in anogenital distance and nipple retention with malformations in androgen-dependent tissues. Toxicological Sciences : An Official Journal of the Society of Toxicology, 62(2), 236–249. https://doi.org/10.1093/toxsci/62.2.236
Mendonca, B., Bloise, W., Arnhold, I., Batista, M., Toledo, S., Drummond, M., Nicolau, W., & Mattar, E. (1987). Male pseudohermaphroditism due to nonsalt-losing 3 beta-hydroxysteroid dehydrogenase deficiency: Gender role change and absence of gynecomastia at puberty. Journal of Steroid Biochemistry, 28(6), 669–675. https://doi.org/10.1016/0022-4731(87)90396-7
Mendonca, B., Inacio, M., Arnhold, I., Costa, E., Bloise, W., Martin, R., Denes, F., Silva, F., Andersson, S., Lindqvist, A., & Wilson, J. (2000). Male pseudohermaphroditism due to 17 beta-hydroxysteroid dehydrogenase 3 deficiency. Diagnosis, psychological evaluation, and management. Medicine, 79(5), 299–309. https://doi.org/10.1097/00005792-200009000-00003
Meuffels, J., Luther-Binoir, I., Daffue, W., Deacon, F., & Mitchell, E. P. (2020). Testicular disorder of sexual development with cryptorchidism, penile hypoplasia and hypospadias in a giraffe (Giraffa camelopardalis giraffa). JOURNAL OF THE SOUTH AFRICAN VETERINARY ASSOCIATION, 91. https://doi.org/10.4102/jsava.v91i0.1971
Murakami, T. (2008). Anatomical Examination of Hypospadias in Cattle. Journal of the Japan Veterinary Medical Association, 61(12), 931–935. https://doi.org/10.12935/jvma1951.61.931
Murashima, A., Kishigami, S., Thomson, A., & Yamada, G. (2015). Androgens and mammalian male reproductive tract development. Biochimica et Biophysica Acta (BBA) - Gene Regulatory Mechanisms, 1849(2), 163–170. https://doi.org/10.1016/j.bbagrm.2014.05.020
Mylchreest, E., Cattley, R., & Foster, P. (1998). Male reproductive tract malformations in rats following gestational and lactational exposure to Di(n-butyl) phthalate: An antiandrogenic mechanism? Toxicological Sciences : An Official Journal of the Society of Toxicology, 43(1), 47–60. https://doi.org/10.1006/toxs.1998.2436
Mylchreest, E., Sar, M., Cattley, R., & Foster, P. (1999). Disruption of androgen-regulated male reproductive development by di(n-butyl) phthalate during late gestation in rats is different from flutamide. Toxicology and Applied Pharmacology, 156(2), 81–95. https://doi.org/10.1006/taap.1999.8643
Nassar, N., Abeywardana, P., Barker, A., & Bower, C. (2010). Parental occupational exposure to potential endocrine disrupting chemicals and risk of hypospadias in infants. Occupational and Environmental Medicine, 67(9), 585–589. https://doi.org/10.1136/oem.2009.048272
Neocleous, V., Sismani, C., Shammas, C., Efstathiou, E., Alexandrou, A., Ioannides, M., Argyrou, M., Patsalis, P., Phylactou, L., & Skordis, N. (2012). Duplication of exons 3-10 of the HSD17B3 gene: A novel type of genetic defect underlying 17 beta-HSD-3 deficiency. GENE, 499(2), 250–255. https://doi.org/10.1016/j.gene.2012.03.031
New, M. (1970). Male pseudohermaphroditism due to 17 alpha-hydroxylase deficiency. The Journal of Clinical Investigation, 49(10), 1930–1941. https://doi.org/10.1172/JCI106412
Nightingale, J., Chaudhary, K. S., Abel, P. D., Stubbs, A. P., Romanska, H. M., Mitchell, S. E., Stamp, G. W. H., & Lalani, E.-N. (2003). Ligand Activation of the Androgen Receptor Downregulates E-Cadherin-Mediated Cell Adhesion and Promotes Apoptosis of Prostatic Cancer Cells. Neoplasia, 5(4), 347–361. https://doi.org/10.1016/S1476-5586(03)80028-3
Nowacka-Woszuk, J., Szczerbal, I., Salamon, S., Kociucka, B., Jackowiak, H., Prozorowska, E., Slaska, B., Rozanska, D., Orzelski, M., Ochota, M., Dzimira, S., Lipiec, M., Nizanski, W., & Switonski, M. (2014). Testicular disorder of sex development in four cats with a male karyotype (38,XY; SRY-positive). Animal Reproduction Science, 151(1–2), 42–48. https://doi.org/10.1016/j.anireprosci.2014.10.001
Okuyama, A., Namiki, M., Koide, T., Itatani, H., Nishimoto, N., Mizutani, S., Sonoda, T., Aono, T., & Matsumoto, K. (1981). Pituitary and gonadal function in prepubertal and pubertal boys with hypospadias. Acta Endocrinologica, 98(3), 464–469. https://doi.org/10.1530/acta.0.0980464
Ostby, J., Kelce, W. R., Lambright, C., Wolf, C. J., Mann, P., & Gray, L. E. (1999). The fungicide procymidone alters sexual differentiation in the male rat by acting as an androgen-receptor antagonist in vivo and in vitro. Toxicology and Industrial Health, 15(1–2), 80–93. https://doi.org/10.1177/074823379901500108
Ostby J, Monosson E, Kelce WR, & Gray, L. J. (1999). Environmental antiandrogens: Low doses of the fungicide vinclozolin alter sexual differentiation of the male rat. Toxicology and Industrial Health, 15(1), 48–64. https://doi.org/10.1177/074823379901500106
Pang, S., Levine, L., Stoner, E., Opitz, J., Pollack, M., Dupont, B., & New, M. (1983). Nonsalt-losing congenital adrenal hyperplasia due to 3 beta-hydroxysteroid dehydrogenase deficiency with normal glomerulosa function. The Journal of Clinical Endocrinology and Metabolism, 56(4), 808–818. https://doi.org/10.1210/jcem-56-4-808
Parks, L. G., Ostby, J. S., Lambright, C. R., Abbott, B.D., Klinefelter, G. R., Barlow, N. J., & Gray LE Jr. (2000). The Plasticizer Diethylhexyl Phthalate Induces Malformations by Decreasing Fetal Testosterone Synthesis during Sexual Differentiation in the Male Rat. Toxicological Sciences, 58(2), 339–349. https://doi.org/10.1093/toxsci/58.2.339
Perrone, L., Criscuolo, T., Sinisi, A., Graziani, M., Manzo, T., Sicuranza, R., Bellastella, A., & Faggiano, M. (1985). Male pseudohermaphroditism due to 3 beta-hydroxysteroid dehydrogenase-isomerase deficiency associated with atrial septal defect. Acta Endocrinologica, 110(4), 532–539. https://doi.org/10.1530/acta.0.1100532
Prahalada, S., Tarantal, A., Harris, G., Ellsworth, K., Clarke, A., Skiles, G., MacKenzie, K., Kruk, L., Ablin, D., Cukierski, M., Peter, C., vanZwieten, M., & Hendrickx, A. (1997). Effects of finasteride, a type 2 5-alpha reductase inhibitor, on fetal development in the rhesus monkey (Macaca mulatta). Teratology, 55(2), 119–131. https://doi.org/10.1002/(SICI)1096-9926(199702)55:2%253C119::AID-TERA1%253E3.0.CO;2-Z
Rabbani, B., Mahdieh, N., Ashtiani, M. H., Setoodeh, A., & A Rabbani. (2012). In silico structural, functional and pathogenicity evaluation of a novel mutation: An overview of HSD3B2 gene mutations. Gene, 503(2), 215–221. https://doi.org/10.1016/j.gene.2012.04.080
Raboch, J., Pondelickova, J., & Starka, L. (1976). Plasma testosterone values in hypopspadiacs. Andrologia, 8(3), 255–258. https://doi.org/10.1111/j.1439-0272.1976.tb02144.x
Radpour R, Rezaee M, Tavasoly A, Solati S, & Saleki A. (2007). Association of long polyglycine tracts (GGN repeats) in exon 1 of the androgen receptor gene with cryptorchidism and penile hypospadias in Iranian patients. Journal of Andrology, 28(1), 164–169. https://doi.org/10.2164/jandrol.106.000927
Ratan, S., Aggarwal, S., Mishra, T., Saxena, A., Yadav, S., Pandey, R., Sharma, A., & Dhanwal, D. (2012). Children with isolated hypospadias have different hormonal profile compared to those with associated anomalies. Journal of Pediatric Endocrinology & Metabolism : JPEM, 25(1), 111–119. https://doi.org/10.1515/jpem.2011.421
Rittmaster, R. S., & Wood, A. J. J. (1994). Finasteride. New England Journal of Medicine, 330(2), 120–125. https://doi.org/10.1056/NEJM199401133300208
Schaufele, F., Carbonell, X., Guerbadot, M., Borngraeber, S., Chapman, M. S., Ma, A. A. K., Miner, J. N., & Diamond, M. I. (2005). The structural basis of androgen receptor activation: Intramolecular and intermolecular amino–carboxy interactions. Proceedings of the National Academy of Sciences, 102(28), 9802–9807. https://doi.org/10.1073/pnas.0408819102
Schlomer, B. J., Feretti, M., Rodriguez, E. J., Blaschko, S., Cunha, G., & Baskin, L. (2013). Sexual differentiation in the male and female mouse from days 0 to 21: A detailed and novel morphometric description. The Journal of Urology, 190(4 Suppl), 1610–1617. https://doi.org/10.1016/j.juro.2013.02.3198
Sharpe, R. M. (2020). Androgens and the masculinization programming window: Human-rodent differences. Biochemical Society Transactions, 48(4), 1725–1735. https://doi.org/10.1042/BST20200200
Sherbet, D., Tiosano, D., Kwist, K., Hochberg, Z., & RJ Auchus. (2003). CYP17 mutation E305G causes isolated 17,20-lyase deficiency by selectively altering substrate binding. The Journal of Biological Chemistry, 278(49), 48563–48569. https://doi.org/10.1074/jbc.M307586200
Simard, J., Luthy, I., Guay, J., Bélanger, A., & Labrie, F. (1986). Characteristics of interaction of the antiandrogen flutamide with the androgen receptor in various target tissues. Molecular and Cellular Endocrinology, 44(3), 261–270. https://doi.org/10.1016/0303-7207(86)90132-2
Sinclair, A., Cao, M., Pask, A., Baskin, L., & Cunha, G. (2017). Flutamide-induced hypospadias in rats: A critical assessment. Differentiation; Research in Biological Diversity, 94, 37–57. https://doi.org/10.1016/j.diff.2016.12.001
Smith, K., Brown, P., & Barr, F. (2012). A Survey of Congenital Reproductive Abnormalities in Rams in Abattoirs in South West England. Reproduction in Domestic Animals, 47(5), 740–745. https://doi.org/10.1111/j.1439-0531.2011.01952.x
Sonne, C., Dietz, R., Born, E. W., Leifsson, P. S., & Andersen, S. (2008). Is there a link between hypospadias and organochlorine exposure in East Greenland sledge dogs (Canis familiaris)? Ecotoxicology and Environmental Safety, 69(3), 391–395. https://doi.org/10.1016/j.ecoenv.2007.09.008
Stamper, M. A., Norton, T., Spodnick, G., Marti, J., & Loomis, M. (1999). Hypospadias in a polar bear (Ursus maritimus). Journal of Zoo and Wildlife Medicine: Official Publication of the American Association of Zoo Veterinarians, 30(1), 141–144.
Svensson, J., Eneroth, P., Gustafsson, J., Ritzén, M., & Stenberg, A. (1979). Reduction of androstenedione by skin in vitro and serum levels of gonadotrophins and androgens in men with hypospadias. The Journal of Endocrinology, 82(3), 395–401. https://doi.org/10.1677/joe.0.0820395
Switonski, M., Dzimira, S., Aleksiewicz, R., Szczerbal, I., Nowacka-Woszuk, J., Krzeminska, P., Deska, T., & Nizanski, W. (2018). Hypospadias Is Not Rare in Dogs: Five New Cases, a Retrospective Study, and a Review of the Literature. Sexual Development : Genetics, Molecular Biology, Evolution, Endocrinology, Embryology, and Pathology of Sex Determination and Differentiation, 12(5), 244–250. https://doi.org/10.1159/000490079
van den Driesche, S., Kilcoyne, K., Wagner, I., Rebourcet, D., Boyle, A., Mitchell, R., McKinnell, C., Macpherson, S., Donat, R., Shukla, C., Jorgensen, A., Meyts, E., Skakkebaek, N., & Sharpe, R. (2017). Experimentally induced testicular dysgenesis syndrome originates in the masculinization programming window. JCI Insight, 2(6), e91204. https://doi.org/10.1172/jci.insight.91204
van den Driesche, S., Shoker, S., Inglis, F., Palermo, C., Langsch, A., & Otter, R. (2020). Systematic comparison of the male reproductive tract in fetal and adult Wistar rats exposed to DBP and DINP in utero during the masculinisation programming window. Toxicology Letters, 335, 37–50. https://doi.org/10.1016/j.toxlet.2020.10.006
Wang, S., Shi, M., Zhu, D., Mathews, R., & Zheng, Z. (2018). External Genital Development, Urethra Formation, and Hypospadias Induction in Guinea Pig: A Double Zipper Model for Human Urethral Development. Urology, 113, 179–186. https://doi.org/10.1016/j.urology.2017.11.002
Wang, S., & Zheng, Z. (2025). Differences in Formation of Prepuce and Urethral Groove During Penile Development Between Guinea Pigs and Mice Are Controlled by Differential Expression of Shh, Fgf10 and Fgfr2. Cells, 14(5), 348. https://doi.org/10.3390/cells14050348
Welsh, M., Saunders, P., Fisken, M., Scott, H., Hutchison, G., Smith, L., & Sharpe, R. (2008). Identification in rats of a programming window for reproductive tract masculinization, disruption of which leads to hypospadias and cryptorchidism. The Journal of Clinical Investigation, 118(4), 1479–1490. https://doi.org/10.1172/JCI34241
Willingham, E., Agras, K., Souza, A. J. de, Konijeti, R., Yucel, S., Rickie, W., Cunha, G., & Baskin, L. (2006). Steroid receptors and mammalian penile development: An unexpected role for progesterone receptor? The Journal of Urology, 176(2), 728–733. https://doi.org/10.1016/j.juro.2006.03.078
Wolf, C., LeBlanc, G., Ostby, J., & Gray, L. J. (2000). Characterization of the period of sensitivity of fetal male sexual development to vinclozolin. Toxicological Sciences : An Official Journal of the Society of Toxicology, 55(1), 152–161. https://doi.org/10.1093/toxsci/55.1.152
Yadav, C., Bajpai, M., Kumar, V., Datta, S., Gupta, P., Ahmed, R., & Banerjee, B. (2011). Polymorphisms in the P450 c17 (17-hydroxylase/17, 20-Lyase) gene: Association with estradiol and testosterone concentration in hypospadias. Urology, 78(4), 902–907. https://doi.org/10.1016/j.urology.2011.04.021
Yu, X., Nassar, N., Mastroiacovo, P., Canfield, M., Groisman, B., Bermejo-Sánchez, E., Ritvanen, A., Kiuru-Kuhlefelt, S., Benavides, A., Sipek, A., Pierini, A., Bianchi, F., Källén, K., Gatt, M., Morgan, M., Tucker, D., Canessa, M. A., Gajardo, R., Mutchinick, O. M., … Agopian, A. J. (2019). Hypospadias Prevalence and Trends in International Birth Defect Surveillance Systems, 1980-2010. European Urology, 76(4), 482–490. https://doi.org/10.1016/j.eururo.2019.06.027
Yucel, S., Liu, W., Cordero, D., Donjacour, A., Cunha, G., & Baskin, L. (2004). Anatomical studies of the fibroblast growth factor-10 mutant, Sonic Hedge Hog mutant and androgen receptor mutant mouse genital tubercle. Advances in Experimental Medicine and Biology, 545, 123–148. https://doi.org/10.1007/978-1-4419-8995-6_8
Zheng, Z., Armfield, B., & Cohn, M. (2015). Timing of androgen receptor disruption and estrogen exposure underlies a spectrum of congenital penile anomalies. Proceedings of the National Academy of Sciences of the United States of America, 112(52), E7194-203. https://doi.org/10.1073/pnas.1515981112