
This AOP is licensed under the BY-SA license. This license allows reusers to distribute, remix, adapt, and build upon the material in any medium or format, so long as attribution is given to the creator. The license allows for commercial use. If you remix, adapt, or build upon the material, you must license the modified material under identical terms.
AOP: 159
Title
Thyroperoxidase inhibition leading to increased mortality via reduced anterior swim bladder inflation
Short name
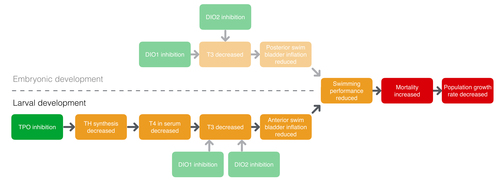
Graphical Representation
Point of Contact
Contributors
- Dries Knapen
- Lucia Vergauwen
- Ann-Cathrin Haigis
Coaches
- Shihori Tanabe
OECD Information Table
| OECD Project # | OECD Status | Reviewer's Reports | Journal-format Article | OECD iLibrary Published Version |
|---|---|---|---|---|
| 1.35 | WPHA/WNT Endorsed | iLibrary link |
This AOP was last modified on April 29, 2023 16:02
Revision dates for related pages
| Page | Revision Date/Time |
|---|---|
| Thyroperoxidase, Inhibition | November 18, 2025 08:29 |
| Decrease, Population growth rate | January 03, 2023 09:09 |
| Thyroid hormone synthesis, Decreased | November 04, 2022 09:25 |
| Thyroxine (T4) in serum, Decreased | October 10, 2022 08:52 |
| Reduced, Anterior swim bladder inflation | October 07, 2022 09:36 |
| Reduced, Swimming performance | September 08, 2021 06:12 |
| Decreased, Triiodothyronine (T3) | October 07, 2022 08:26 |
| Increased Mortality | July 08, 2022 07:32 |
| Thyroperoxidase, Inhibition leads to TH synthesis, Decreased | November 04, 2022 09:27 |
| TH synthesis, Decreased leads to T4 in serum, Decreased | October 10, 2022 08:56 |
| T4 in serum, Decreased leads to Decreased, Triiodothyronine (T3) | October 10, 2022 09:02 |
| Decreased, Triiodothyronine (T3) leads to Reduced, Anterior swim bladder inflation | October 07, 2022 09:44 |
| Reduced, Anterior swim bladder inflation leads to Reduced, Swimming performance | October 07, 2022 09:48 |
| Reduced, Swimming performance leads to Increased Mortality | September 08, 2021 10:05 |
| Increased Mortality leads to Decrease, Population growth rate | July 08, 2022 08:29 |
| Thyroperoxidase, Inhibition leads to T4 in serum, Decreased | November 18, 2025 08:50 |
| T4 in serum, Decreased leads to Reduced, Anterior swim bladder inflation | October 10, 2022 09:13 |
| Methimazole | November 29, 2016 18:42 |
| Mercaptobenzothiazole | November 29, 2016 18:42 |
| Propylthiouracil | November 29, 2016 18:42 |
Abstract
This AOP describes the sequence of events leading from thyroperoxidase inhibition to increased mortality via reduced anterior swim bladder inflation. The enzyme thyroperoxidase (TPO) is essential for the synthesis of thyroxine (T4) and triiodothyronine (T3) in the thyroid follicles. Inhibition of TPO reduces thyroid hormone (TH) levels. Thyroid hormones are critical in regulating developmental processes and thyroid hormone disruption can interfere with normal development. Swim bladder inflation is known to be under TH control (Brown et al., 1988; Liu and Chan, 2002). Many fish species have a swim bladder which is a gas-filled organ that typically consists of two chambers (Robertson et al., 2007). The posterior chamber inflates during early development in the embryonic phase, while the anterior chamber inflates during late development in the larval phase. Both the posterior and the anterior chamber have an important role in regulating buoyancy, and the anterior chamber has an additional role in hearing (Robertson et al., 2007).
This AOP describes how inhibition of TPO results in decreased synthesis of THs in the thyroid follicles. This reduces the availability of T4 for conversion to the more biologically active T3. Reduced T3 levels prohibit normal inflation of the anterior swim bladder chamber. Due to its role in regulating buoyancy, this results in reduced swimming performance. Since reduced swimming performance results in a decreased ability to forage and avoid predators, this reduces chances of survival. The final adverse outcome is a decrease of the population growth rate. Since many AOPs eventually lead to this more general adverse outcome at the population level, the more specific and informative adverse outcome at the organismal level, increased mortality, is used in the AOP title. Support for this AOP is mainly based on chemical exposures in zebrafish and fathead minnows (Nelson et al., 2016; Godfrey et al., 2017; Stinckens et al., 2016, 2020). Convincing evidence comes from a TPO knockout study in zebrafish confirming that anterior inflation was reduced (Fang et al. 2022). Additional evidence of a link between reduced TH synthesis and reduced anterior chamber inflation is available from a study where a mutation was inserted in the gene coding for dual oxidase, another enzyme that is important for TH synthesis since it provides hydrogen peroxide for iodide oxidation (Chopra et al., 2019).
This AOP is part of a larger AOP network describing how decreased synthesis and/or decreased biological activation of THs leads to incomplete or improper inflation of the swim bladder, leading to reduced swimming performance, increased mortality and decreased population trajectory (Knapen et al., 2018; Knapen et al., 2020; Villeneuve et al., 2018). Apart from the upstream part, the current AOP is identical to the corresponding AOPs leading from DIO1 and DIO2 inhibition to increased mortality via anterior swim bladder inflation (https://aopwiki.org/aops/156, https://aopwiki.org/aops/158).
AOP Development Strategy
Context
The larger AOP network describing the effect of deiodinase and thyroperoxidase inhibition on swim bladder inflation consists of 5 AOPs:
- Deiodinase 2 inhibition leading to increased mortality via reduced posterior swim bladder inflation: https://aopwiki.org/aops/155
- Deiodinase 2 inhibition leading to increased mortality via reduced anterior swim bladder inflation: https://aopwiki.org/aops/156
- Deiodinase 1 inhibition leading to increased mortality via reduced posterior swim bladder inflation : https://aopwiki.org/aops/157
- Deiodinase 1 inhibition leading to increased mortality via reduced anterior swim bladder inflation : https://aopwiki.org/aops/158
- Thyroperoxidase inhibition leading to increased mortality via reduced anterior swim bladder inflation: https://aopwiki.org/aops/159
The development of these AOPs was mainly based on a series of dedicated experiments (using a set of reference chemicals as prototypical stressors) in zebrafish and fathead minnow that form the core of the empirical evidence. Specific literature searches were used to add evidence from other studies, mainly in zebrafish and fathead minnow. No systematic review approach was applied.
Strategy
Summary of the AOP
Events:
Molecular Initiating Events (MIE)
Key Events (KE)
Adverse Outcomes (AO)
| Type | Event ID | Title | Short name |
|---|
| MIE | 279 | Thyroperoxidase, Inhibition | Thyroperoxidase, Inhibition |
| KE | 277 | Thyroid hormone synthesis, Decreased | TH synthesis, Decreased |
| KE | 281 | Thyroxine (T4) in serum, Decreased | T4 in serum, Decreased |
| KE | 1003 | Decreased, Triiodothyronine (T3) | Decreased, Triiodothyronine (T3) |
| KE | 1007 | Reduced, Anterior swim bladder inflation | Reduced, Anterior swim bladder inflation |
| KE | 1005 | Reduced, Swimming performance | Reduced, Swimming performance |
| AO | 351 | Increased Mortality | Increased Mortality |
| AO | 360 | Decrease, Population growth rate | Decrease, Population growth rate |
Relationships Between Two Key Events (Including MIEs and AOs)
| Title | Adjacency | Evidence | Quantitative Understanding |
|---|
| Thyroperoxidase, Inhibition leads to TH synthesis, Decreased | adjacent | High | Low |
| TH synthesis, Decreased leads to T4 in serum, Decreased | adjacent | Moderate | Low |
| T4 in serum, Decreased leads to Decreased, Triiodothyronine (T3) | adjacent | Moderate | Moderate |
| Decreased, Triiodothyronine (T3) leads to Reduced, Anterior swim bladder inflation | adjacent | Moderate | Moderate |
| Reduced, Anterior swim bladder inflation leads to Reduced, Swimming performance | adjacent | Moderate | Low |
| Reduced, Swimming performance leads to Increased Mortality | adjacent | Moderate | Low |
| Increased Mortality leads to Decrease, Population growth rate | adjacent | Moderate | Moderate |
| Thyroperoxidase, Inhibition leads to T4 in serum, Decreased | non-adjacent | High | Low |
| T4 in serum, Decreased leads to Reduced, Anterior swim bladder inflation | non-adjacent | Moderate | Moderate |
Network View
Prototypical Stressors
Life Stage Applicability
| Life stage | Evidence |
|---|---|
| Larvae | High |
Taxonomic Applicability
Sex Applicability
| Sex | Evidence |
|---|---|
| Unspecific | Moderate |
Overall Assessment of the AOP

The attached document includes:
- Support for biological plausibility of KERs
- Support for essentiality of KEs
- Empirical support for KERs
- Dose and temporal concordance table covering the larger AOP network
Overall, the weight of evidence for the sequence of key events laid out in the AOP is moderate to high. Nonetheless, the exact underlying mechanism of TH disruption leading to impaired swim bladder inflation is not understood.
Domain of Applicability
Taxonomic: Organogenesis of the swim bladder begins with an evagination from the gut. In physostomous fish, a connection between the swim bladder and the gut is retained. In physoclystous fish, once initial inflation by gulping atmospheric air at the water surface has occurred, the swim bladder is closed off from the digestive tract and swim bladder volume is regulated by gas secretion into the swim bladder (Woolley and Qin, 2010). This AOP is currently mainly based on experimental evidence from studies on zebrafish and fathead minnows, physostomous fish with a two-chambered swim bladder. This AOP is not applicable to fish that do not have a second swim bladder chamber that inflates during larval development, e.g., the Japanese rice fish or medaka (Oryzias latipes).
Life stage: The current AOP is applicable to the larval life stage, the period in which the anterior chamber of the swim bladder inflates (21 days post fertilization in zebrafish).
Sex: All key events in this AOP are plausibly applicable to both sexes. Sex differences are not often investigated in tests using early life stages of fish. For zebrafish and fathead minnow, it is currently unclear whether sex-related differences are important in determining the magnitude of the changes across the sequence of events in this AOP. Different fish species have different sex determination and differentiation strategies. Zebrafish do not have identifiable heteromorphic sex chromosomes and sex is determined by multiple genes and influenced by the environment (Nagabhushana and Mishra, 2016). Zebrafish are undifferentiated gonochorists since both sexes initially develop an immature ovary (Maack and Segner, 2003). Immature ovary development progresses until approximately the onset of the third week. Later, in female fish immature ovaries continue to develop further, while male fish undergo transformation of ovaries into testes. Final transformation into testes varies among male individuals, however finishes usually around 6 weeks post fertilization. Since the anterior chamber inflates around 21 days post fertilization in zebrafish, sex differences are expected to play a minor role in the current AOP. Fathead minnow gonad differentiation also occurs during larval development. Fathead minnows utilize a XY sex determination strategy and markers can be used to genotype sex in life stages where the sex is not yet clearly defined morphologically (Olmstead et al., 2011). Ovarian differentiation starts at 10 dph followed by rapid development (Van Aerle et al., 2004). At 25 dph germ cells of all stages up to the primary oocytes stage were present and at 120 dph, vitellogenic oocytes were present. The germ cells (spermatogonia) of the developing testes only entered meiosis around 90–120 dph. Mature testes with spermatozoa are present around 150 dph. Since the anterior chamber inflates around 14 days post fertilization (9 dph) in fathead minnows, sex differences are expected to play a minor role in the current AOP.
Essentiality of the Key Events
Overall, the confidence in the supporting data for essentiality of KEs within the AOP is moderate. There is indirect evidence that reduced thyroid hormone synthesis causes reduced anterior swim bladder inflation from a study where a similar MIE was targeted: Chopra et al. (2019) showed that knockdown of dual oxidase, another enzyme that is important for TH synthesis since it provides hydrogen peroxide for iodide oxidation, reduced anterior swim bladder inflation. It should be noted that dual oxidase also plays a role in oxidative stress. Additionally, there is indirect evidence from deiodinase knockdowns supporting the downstream part of the AOP linking decreased T3 levels to reduced swim bladder inflation (targeted at posterior chamber inflation, not specifically at anterior chamber inflation, see AOPs 155-158). There is also evidence that alleviation of the effect on anterior chamber inflation reduces the effect on swimming performance.
Evidence Assessment
Biological plausibility: see Table. Overall, the weight of evidence for the biological plausibility of the KERs in the AOP is moderate since there is empirical support for an association between the sets of KEs and the KERs are plausible based on analogy to accepted biological relationships, but scientific understanding is not completely established.
Empirical support: see Table. Overall, the empirical support for the KERs in the AOP is moderate since dependent changes in sets of KEs following exposure to several specific stressors has been demonstrated, with limited evidence for dose and temporal concordance and some uncertainties.
Known Modulating Factors
| Modulating Factor (MF) | Influence or Outcome | KER(s) involved |
|---|---|---|
Quantitative Understanding
There is some level of quantitative understanding that can form the basis for development of a quantitative AOP. Quantitative relationships between reduced T4 and reduced T3, and between reduced T3 and reduced anterior chamber inflation were established. The latter is particularly critical for linking impaired swim bladder inflation to TH disruption.
Considerations for Potential Applications of the AOP (optional)
A growing number of environmental pollutants are known to adversely affect the thyroid hormone system, and major gaps have been identified in the tools available for the identification, and the hazard and risk assessment of these thyroid hormone disrupting chemicals. Villeneuve et al. (2014) discussed the relevance of swim bladder inflation as a potential key event and endpoint of interest in fish tests. Knapen et al. (2020) provide an example of how the adverse outcome pathway (AOP) framework and associated data generation can address current testing challenges in the context of fish early-life stage tests, and fish tests in general. While the AOP is only applicable to fish, some of the upstream KEs are relevant across vertebrates. The taxonomic domain of applicability call of the KEs can be found on the respective pages. A suite of assays covering all the essential biological processes involved in the underlying toxicological pathways can be implemented in a tiered screening and testing approach for thyroid hormone disruption in fish, using the levels of assessment of the OECD’s Conceptual Framework for the Testing and Assessment of Endocrine Disrupting Chemicals as a guide. Specifically, for this AOP, thyroperoxidase inhibition can be assessed using an in chemico assay, measurements of T4 and T3 levels could be added to the Fish Embryo Acute Toxicity (FET) test (OECD TG 236), the Fish Early Life Stage Toxicity (FELS) Test (OECD TG210) and the Fish Sexual Development Test (FSDT), and assessments of anterior chamber inflation and swimming performance could be added to the FELS Test and FSDT.
Thyroid hormone system disruption causes multiple unspecific effects. Addition of TH measurements could aid in increasing the diagnostic capacity of a battery of endpoints since they are specific to the TH system. A battery of endpoints would ideally include the MIE, the AO and TH levels as the causal link. It is also in this philosophy that TH measurements are currently being considered as one of the endpoints in project 2.64 of the OECD TG work plan, “Inclusion of thyroid endpoints in OECD fish Test Guidelines”. While thyroid hormone measurements showed low levels of variation and were highly predictive of downstream effects in dedicated experiments to support this AOP, more variability may be present in other studies. Because of the rapid development in fish, it is important to compare thyroid hormone levels within specific developmental stages. For example, clear changes in thyroid hormone levels have been observed in zebrafish at 5, 14, 21 and 32 dpf (Stinckens et al., 2016; Stinckens et al., 2020) and in fathead minnows at 4, 6, 10, 14, 18 and 21 dpf (Nelson et al., 2016; Cavallin et al., 2017) using liquid chromatography tandem mass spectrometry (LC−MS/MS).
References
Brown, C.L., Doroshov, S.I., Nunez, J.M., Hadley, C., Vaneenennaam, J., Nishioka, R.S., Bern, H.A., 1988. MATERNAL TRIIODOTHYRONINE INJECTIONS CAUSE INCREASES IN SWIMBLADDER INFLATION AND SURVIVAL RATES IN LARVAL STRIPED BASS, MORONE-SAXATILIS. Journal of Experimental Zoology 248, 168-176.
Chopra, K., Ishibashi, S., Amaya, E., 2019. Zebrafish duox mutations provide a model for human congenital hypothyroidism. Biology Open 8.
Flores MV, Crawford KC, Pullin LM, Hall CJ, Crosier KE, Crosier PS. 2010. Dual oxidase in the intestinal epithelium of zebrafish larvae has anti-bacterial properties. Biochemical and Biophysical Research Communications. 400(1):164-168.
Godfrey, A., Hooser, B., Abdelmoneim, A., Horzmann, K.A., Freemanc, J.L., Sepulveda, M.S., 2017. Thyroid disrupting effects of halogenated and next generation chemicals on the swim bladder development of zebrafish. Aquatic Toxicology 193, 228-235.
Knapen, D., Angrish, M.M., Fortin, M.C., Katsiadaki, I., Leonard, M., Margiotta-Casaluci, L., Munn, S., O'Brien, J.M., Pollesch, N., Smith, L.C., Zhang, X.W., Villeneuve, D.L., 2018. Adverse outcome pathway networks I: Development and applications. Environmental Toxicology and Chemistry 37, 1723-1733.
Knapen, D., Stinckens, E., Cavallin, J.E., Ankley, G.T., Holbech, H., Villeneuve, D.L., Vergauwen, L., 2020. Toward an AOP Network-Based Tiered Testing Strategy for the Assessment of Thyroid Hormone Disruption. Environmental Science & Technology 54, 8491-8499.
Liu, Y.W., Chan, W.K., 2002. Thyroid hormones are important for embryonic to larval transitory phase in zebrafish. Differentiation 70, 36-45.
Maack, G., Segner, H., 2003. Morphological development of the gonads in zebrafish. Journal of Fish Biology 62, 895-906.
Nagabhushana A, Mishra RK. 2016. Finding clues to the riddle of sex determination in zebrafish. Journal of Biosciences. 41(1):145-155.
Nelson, K., Schroeder, A., Ankley, G., Blackwell, B., Blanksma, C., Degitz, S., Flynn, K., Jensen, K., Johnson, R., Kahl, M., Knapen, D., Kosian, P., Milsk, R., Randolph, E., Saari, T., Stinckens, E., Vergauwen, L., Villeneuve, D., 2016. Impaired anterior swim bladder inflation following exposure to the thyroid peroxidase inhibitor 2-mercaptobenzothiazole part I: Fathead minnow. Aquatic Toxicology 173, 192-203.
Niethammer P, Grabher C, Look AT, Mitchison TJ. 2009. A tissue-scale gradient of hydrogen peroxide mediates rapid wound detection in zebrafish. Nature. 459(7249):996-U123.
Olmstead AW, Villeneuve DL, Ankley GT, Cavallin JE, Lindberg-Livingston A, Wehmas LC, Degitz SJ. 2011. A method for the determination of genetic sex in the fathead minnow, pimephales promelas, to support testing of endocrine-active chemicals. Environmental Science & Technology. 45(7):3090-3095.
Robertson, G.N., McGee, C.A.S., Dumbarton, T.C., Croll, R.P., Smith, F.M., 2007. Development of the swimbladder and its innervation in the zebrafish, Danio rerio. Journal of Morphology 268, 967-985.
Stinckens, E., Vergauwen, L., Blackwell, B.R., Anldey, G.T., Villeneuve, D.L., Knapen, D., 2020. Effect of Thyroperoxidase and Deiodinase Inhibition on Anterior Swim Bladder Inflation in the Zebrafish. Environmental Science & Technology 54, 6213-6223.
Stinckens, E., Vergauwen, L., Schroeder, A., Maho, W., Blackwell, B., Witters, H., Blust, R., Ankley, G., Covaci, A., Villeneuve, D., Knapen, D., 2016. Impaired anterior swim bladder inflation following exposure to the thyroid peroxidase inhibitor 2-mercaptobenzothiazole part II: Zebrafish. Aquatic Toxicology 173, 204-217.
van Aerle R, Runnalls TJ, Tyler CR. 2004. Ontogeny of gonadal sex development relative to growth in fathead minnow. Journal of Fish Biology. 64(2):355-369.
Villeneuve, D., Angrish, M., Fortin, M., Katsiadaki, I., Leonard, M., Margiotta-Casaluci, L., Munn, S., O'Brien, J., Pollesch, N., Smith, L., Zhang, X., Knapen, D., 2018. Adverse Outcome Pathway Networks II: Network Analytics. Environ Toxicol Chem doi: 10.1002/etc.4124.
Villeneuve, D., Volz, D.C., Embry, M.R., Ankley, G.T., Belanger, S.E., Leonard, M., Schirmer, K., Tanguay, R., Truong, L., Wehmas, L., 2014. Investigating alternatives to the fish early-life stage test: a strategy for discovering and annotating adverse outcome pathways for early fish development. Environmental Toxicology and Chemistry 33, 158-169.
Woolley, L.D., Qin, J.G., 2010. Swimbladder inflation and its implication to the culture of marine finfish larvae. Reviews in Aquaculture 2, 181-190.
Xu JP, Zhang RT, Zhang T, Zhao G, Huang Y, Wang HL, Liu JX. 2017. Copper impairs zebrafish swimbladder development by down-regulating wnt signaling. Aquatic Toxicology. 192:155-164.
Zhou XY, Zhang T, Ren L, Wu JJ, Wang WM, Liu JX. 2016. Copper elevated embryonic hemoglobin through reactive oxygen species during zebrafish erythrogenesis. Aquatic Toxicology. 175:1-11.